Back to: CHEMISTRY SS2
Welcome to Class !!
We are eager to have you join us !!
In today’s Chemistry class, We will be learning about Hydrocarbons. We hope you enjoy the class!

CONTENT
- Unique Nature of Carbon.
- Characteristics Features of Organic Compounds
- Classification of Hydrocarbons.
- Definition of Terms used in Organic Chemistry
Organic chemistry is the chemistry of carbon compounds with the exception of compounds such as carbon (II) oxide, carbon (IV) oxide the trioxocarbonate (IV). Carbon has a unique ability to form numerous organic compounds because it has the ability to catenate. Catenation is the ability of atoms of an element to form bonds between its own atoms and produce long-chain structures.
All organic compounds contain carbon as the main element together with one or more other elements such as hydrogen, oxygen, chlorine, nitrogen and sulphur.
UNIQUE NATURE OF CARBON
- The valency of carbon: the electronic configuration of carbon is as follows:
C = 6: 1s2 2s1 2px1 2py1 2pz1
Carbon forms covalent bonds and after bond formation, it has neither vacant orbital nor lone pair of electrons. This makes many carbon compounds chemically stable.
- The bond between carbon and hydrogen is non-polar. Thus, hydrogen atoms attached to carbon do not weaken carbon-carbon bonds.
- The types of orbital hybridization available to carbon: carbon can form three types of hybridizations: SP3, SP2 and SP hybridization. Hence, it has the ability to form single, double or triple covalent bonds between its atoms.
- A large amount of energy is required to break a carbon single bond.
CHARACTERISTICS FEATURE OF ORGANIC COMPOUNDS
- Organic compounds are covalent in nature.
- They are non-polar substances and are insoluble in polar solvents.
- They have low melting and boiling points.
- They are highly flammable.
- Their reactions are relatively slow compared to inorganic chemical reactions.
CLASSIFICATION OF ORGANIC COMPOUNDS
Organic compounds are classified into Aliphatic and Aromatic compounds.
Aliphatic Compounds: These are compounds whose molecules are composed of chains of carbon atoms. They can be
- Straight chain compounds e.g. pentane
- Branched-chain compounds e.g. 2-methyl butane
Straight and branched-chain aliphatic compounds exist as open chain and are called ACYCLIC compounds. Aliphatic compounds which exist as the closed chain are called the CYCLIC compounds e.g. cyclopropane.
Aromatic Compounds: Benzene, C6H6, is a typical aromatic compound. Other aromatics compounds are derivatives of benzene e.g. C6H5OH.
EVALUATION
- List four reasons why carbon forms numerous organic compounds
- State five characteristic features of organic compounds.
DEFINITION OF TERMS USED INORGANIC CHEMISTRY
HOMOLOGOUS SERIES
Homologous series is a family of organic compounds which follows a regular structural pattern and in which each successive member differs in its molecular formula by –CH2– group.
The simplest series of compounds in organic chemistry is the Alkane series. The general molecular formula of the alkane series is CnH2n+2. It is the parent series from which every other series is obtained. Other homologous series include the Alkenes, Alkynes, Alkanols, Alkanoic acids, etc.
CHARACTERISTICS OF HOMOLOGOUS SERIES
- All members conform to general molecular formula, e.g for alkanes, CnH2n+2
- Each successive member differs in the molecular formula by – CH2– group.
- All members undergo similar chemical reactions.
- The physical properties of members change gradually along the series.
- All members are prepared by the same method.
ALKYL AND FUNCTIONAL GROUPS
Alkyl groups: Alkyl groups are all groups derived from the alkanes by the loss of a hydrogen atom. Alkyl groups have a general formula of CnH2n+1. They are named after the parent alkane by replacing the ending –ane by –yl. The alkyl group derived from the first two members of the parent alkane series are:
Parent alkane Alkyl group Formula
Methane, CH4 Methyl -CH3
Ethane, C2H6 Ethyl -C2H5
Functional groups: The substituent of a hydrogen atom in the alkane series to form the alkyl group determines the chemical properties of the compound formed thereafter. This substituent is called FUNCTIONAL GROUP.
A functional group is an atom, a radical or a bond common to a homologous series and which determines the chemical properties of the series.
Examples of functional groups include Hydroxyl group -OH, amino group NH2, carboxyl group -COO, double and triple bonds.
Alkyl group in a compound determines the physical properties of the compound; while functional group determine the chemical properties of the compound.
EVALUATION
- Define a homologous series.
- Define a functional group and give two examples.
SATURATED AND UNSATURATED COMPOUNDS
Saturated compounds are compounds containing atoms joined together by a single covalent bond. Alkanes are saturated compounds, e.g. ethane, C2H6
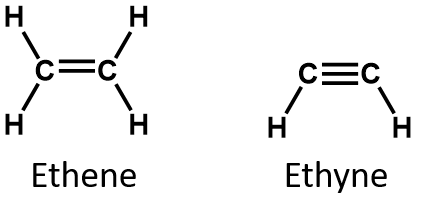
Unsaturated compounds are compounds containing atoms joined together by double or triple bonds. Alkenes and alkynes are unsaturated compounds,
e.g. Ethene, C2H4 Ethyne C2H2
FORMULAE OF ORGANIC COMPOUNDS
Organic compounds are characterized by the following formulae
- Empirical formula
- Molecular formula
- Structural formula
-Empirical Formula is the simplest formula which indicates the component elements and ratio of the combination of atoms in a compound.
– Molecular Formula is a chemical formula of a compound which indicates the actual number of atoms of each element in a compound.
-Structural Formula is a formula which indicates how atoms are arranged within the molecule of a substance.
A structural formula can be
- Open structural formula
- Condensed structural formula
Open structure Condensed structure
CH3CH3
GENERAL EVALUATION/REVISION
- Differentiate between saturated and unsaturated compounds.
- Write the open and condensed structural formula of pentane [C5H12].
- Define the following terms: Homologous series and functional group.
- Determine the oxidation number of Cl in each of the following compounds and give the IUPAC name of the compound (a) NaOCl (b) KClO3
- Split the following redox equations into oxidation and reduction half equation
(a) Cu(s) + 2Ag+(aq) → Cu2+(aq) + 2Ag(s)
(b) Cl2(g) + 2I–(aq) → 2Cl– + I2(s)
READING ASSIGNMENT
New School Chemistry for Senior Secondary School by O .Y. Ababio (6th edition), pages 514-520
WEEKEND ASSIGNMENT
SECTION A: Write the correct option ONLY.
- An exceptionally large number of carbon compounds is essentially due to the ability of
(a) carbon to catenate liberally
(b) various groups to catenate
(c) nitrogen, hydrogen, phosphorus and the halogens to catenate with themselves
(d) hydrocarbons to dominate other groups.
- Which of the following is not a characteristics feature of organic compounds? (a). Covalent in nature (b). They dissolve in all polar solvents (c) Low melting and boiling points (d) Highly flammable
- Functional groups in organic compounds (a) determine the chemical properties of the homologous series (b) does not modify the other when they are more than one in a molecule (c) have a general formula which may include the functional group (d) are responsible for the physical properties.
- Homologues have the same (a) empirical formula (b) structural formula (c) general formula (d) molecular formula
- The four main classes of hydrocarbons are (a).methane, ethene, ethyne and benzene (b) ethane, ethene, ethyne and toluene (c) cycloalkane, cycloalkene, alkynes and arenes (d) alkanes, alkenes, alkynes and aromatics
SECTION B
- Define the following terms: a. Functional group b. Homologous series
- Write the open chain structure of the following
- CH3C(CH3)2CH2CH(CH3)CH2CH3
- (CH3)2CHCH2CH(CH2Cl)CH3
- CH3C(Br)2CH2CH3
We have come to the end of this class. We do hope you enjoyed the class?
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
In our next class, we will be learning about Saturated Hydrocarbons. We are very much eager to meet you there.

How do we submit our assignment
How do we submit the reading assignment and the reading assignment