Back to: CHEMISTRY SS2
Welcome to Class !!
We are eager to have you join us !!
In today’s Chemistry class, We will be learning about Ionic Theory. We hope you enjoy the class!
CONTENT
Distinguish between:
- Electrovalent and Covalent Compounds.
- Electrolytes and Non-electrolytes.
- Weak and Strong Electrolytes.
- Conductors and Non –conductors.
IONIC THEORY
The ionic theory was first presented by Arrhenius to explain electrolysis. The ionic theory proposed that when an electrolyte is melted or dissolved in water, some if not all, the molecules of the substance dissociate into freely moving charged particles called ions. The process of dissociation into ions is known as ionization.
The metallic ions, ammonium ions, NH4+, and hydrogen ions, H+, are positively charged while the nonmetallic ions and hydroxide ions are negatively charged. When an electric current is passed through an electrolyte, the free ions lose their random movement. The positive ions become attracted to the cathode (negative electrode) and are known as cations (i.e. cathode ions). The negative ions move towards the anode (positive electrode) and are called anions. (i.e. anode ions). Therefore, the current through the electrolyte is carried by the movement of ions to the electrodes, and not by flow of electrons in the electrolyte.
Arrhenius version of the ionic theory has been modified and replaced by the modern ionic theory. The modern ionic theory proposes that an electrolyte consists of oppositely charged ions even in the solid-state and such ions are pulled away from one another either as a result of the heat applied when the solid melts or with the help of the solvent molecules when the solid dissolves.
ARRHENIUS THEORY: NaCl(s) Na+(aq) + Cl–(aq)
MODERN THEORY: Na+Cl–(s) Na+(aq) + Cl–(aq)
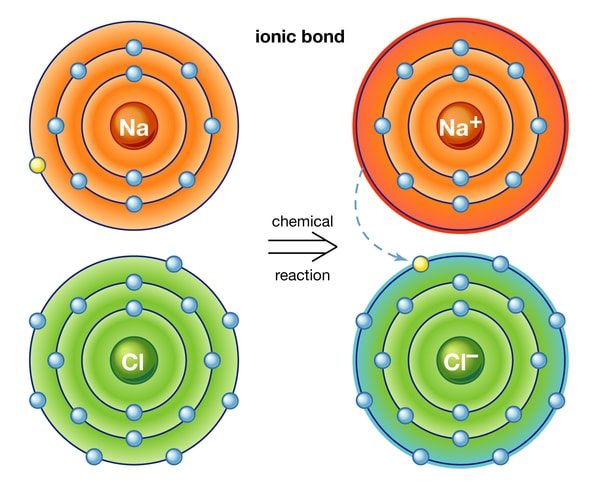
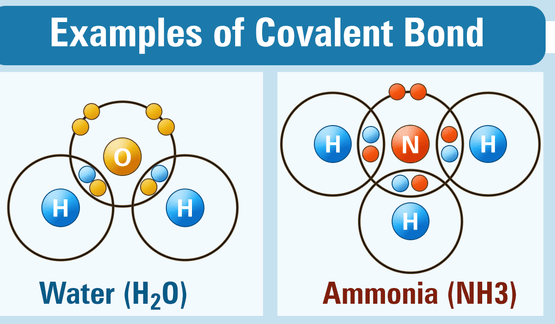
ELECTROVALENT AND COVALENT COMPOUND
Electrovalent compounds or Ionic are formed by the complete transfer of an electron(s) from a metal to a non-metal and they are made up of ions while covalent compounds are formed by the sharing of electrons between the two atoms combining together and they are made up of molecules.
CHARACTERISTICS OF ELECTROVALENT COMPOUNDS
- Electrovalent compounds are usually crystalline solids at room temperature.
- Electrovalent compounds are usually soluble in polar solvents like water but insoluble in non-polar solvents like tetrachloromethane.
- Electrovalent compounds generally have high melting and boiling points.
- Electrovalent compounds are good conductors of electricity in the molten state and in aqueous solutions but insulators in the solid-state.
CHARACTERISTICS OF COVALENT COMPOUNDS
- Covalent compounds are usually liquids or gases.
- Covalent compounds are soluble in non-polar solvents like benzene or carbon tetrachloride and insoluble in polar solvents like water.
- Covalent compounds generally have low melting and boiling points.
- Covalent compounds are bad conductors of electricity.
EVALUATION
- Explain the ionic theory.
- Distinguish between electrovalent compounds and covalent compounds.
ELECTROLYTES AND NON-ELECTROLYTES
An electrolyte is any salt or molecule that ionizes when dissolved in solution and the solution can conduct electricity while a non-electrolyte does not dissociate in solution and does not conduct electricity. Electrolytes can conduct electricity because when salt dissolves, its dissociated ions can move freely in solution, allowing the flow of -charges.
Examples of electrolytes are molten or aqueous sodium chlorine, mineral acids like tetraoxosulphate (VI) acid, hydrochloric acid, potassium hydroxide etc.
Examples of non-electrolytes are ethanol, benzene, sugar, urea, etc.
WEAK AND STRONG ELECTROLYTES
A strong electrolyte is one which ionizes completely in solution and conducts electric current readily example: tetraoxosulphate (VI) acid, sodium hydroxide, sodium chloride etc.; while weak electrolyte is one which ionizes partially in solution and does not conduct electric current readily example ethanoic acid, aqueous ammonia, water and so on.
The conductivity of strong electrolytes decreases slightly with increasing concentration; however, the conductivity of weak electrolytes increases with decreasing concentration.
CONDUCTORS AND NON–CONDUCTORS
Any substance that allows the passage of electricity through it is called a conductor; while any substance that does not allow the passage of electricity is known as non-conductor or insulator.
Conductors are mostly metals. Glass, porcelain, plastic, rubber and diamond are examples of non–conductive materials
GENERAL EVALUTION/REVISION
- Distinguish between conductor and non-conductor giving examples.
- Differentiate between a strong electrolyte and weak electrolyte giving examples.
- State THREE differences between electrovalent and covalent compounds.
- Write the electronic configuration of the following atom/ion: S2-, Al3+, Fe, Cl and Ar.
- Two isotopes of the element Z with mass numbers 18 and 20 are in the ratio of 1:2. Determine the relative atomic mass of Z.
READING ASSIGNMENT
New School Chemistry for Senior Secondary School (6th edition) by O. Y. Ababio, pages 200-201
WEEKEND ASSIGNMENT
SECTION A: Write the correct option ONLY
- Which of the following liquids is a good conductor of electricity? A. Methylbenzene B. Deionized water C. Mercury D. Sucrose solution
- Potassium chloride cannot conduct an electric current in the solid-state because it A. does not contain mobile ions. B. is very soluble in water. C. is an electrovalent compound D. is a neutral salt.
- What happens to the conductivity of a strong electrolyte as its concentration reduces? It A. increases B. decreases C. is unaffected D. reduces to zero
- The current carriers that are responsible for the conductance of electrolytes are A. hydrated electrons B. hydrated ions C. electrons D. ions
- The current carriers that are responsible for the conductance of conductors are A. hydrated electrons B. hydrated ions C. electrons D. ions
SECTION B
- Differentiate between strong electrolyte and weak electrolyte giving examples.
- State THREE differences between electrovalent and covalent compounds.
We have come to the end of this class. We do hope you enjoyed the class?
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
In our next class, we will be learning about Electrolysis. We are very much eager to meet you there.

Thank you very much for this. I have Post UTME on Monday and this as helped improve my confidence.
thank you so much the note I s really interesting.. you are welcome