Back to: CHEMISTRY SS2
Welcome to Class !!
We are eager to have you join us !!
In today’s Chemistry class, We will be learning about the element Sulphur. We hope you enjoy the class!
CONTENT
- General Properties of Sulphur Group.
- Electronic Structure of Sulphur Group.
- Extraction of Sulphur.
- Allotropes of Sulphur.
- Uses of Sulphur
GENERAL PROPERTIES OF THE SULPHUR GROUP (GROUP VI ELEMENTS)
The group VI elements include Oxygen, Sulphur, Selenium, Tellurium and Polonium.
- Metallic property increases down the group. Oxygen and sulphur are non-metal; selenium and tellurium are metalloids; while polonium is a metal.
- All the elements are solid except oxygen which is a gas at room temperature.
- Oxygen and sulphur exhibit allotropy.
- They have six electrons in their outermost shell. Hence their oxidation number is -2; though sulphur can exhibit -4 and -6 states in some compounds.
- Electronegativity decreases down the group. Thus, oxygen is a good oxidizing agent.
ELECTRONIC STRUCTURE OF SULPHUR GROUP
Members of the sulphur family include Oxygen, Sulphur, Selenium, Tellurium and Polonium. Their electronic configurations are shown below:
Oxygen = 8: 1s2 2s2 2p4
Sulphur = 16: 1s2 2s2 2p6 3s2 3p4
Selenium= 34: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p4
Tellurium = 52: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p4
Polonium = 84: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 5d10 5f14 6s2 6p4
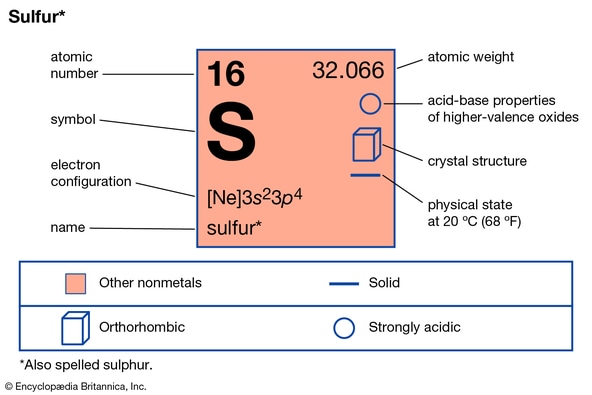
SULPHUR
Sulphur is an element. It occurs freely as deposits and in combined state as sulphide and as tetraoxosulphate (IV).
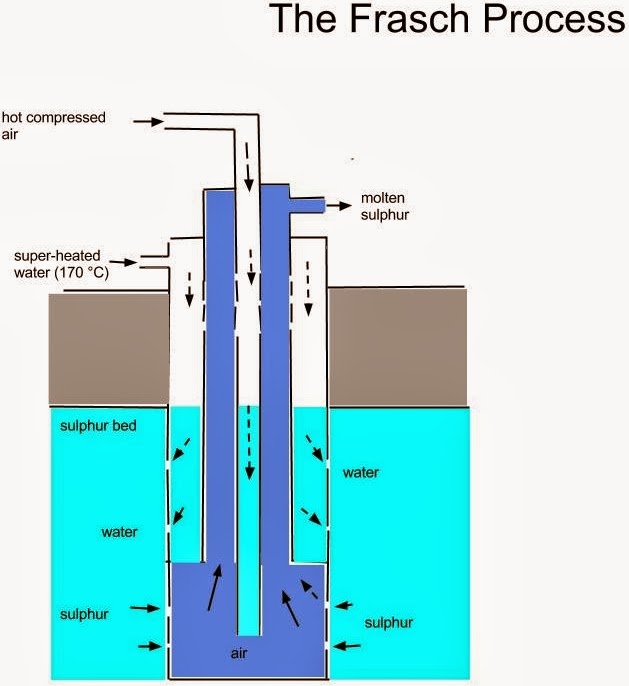
EXTRACTION OF SULPHUR
Sulphur is extracted from underground by Frasch process. A three concentric sulphur pump is driven down a hole dug to the sulphur bed. The solid sulphur is melted at 115oC by superheated water at 170oC and 10atms. The molten sulphur is forced out by hot compressed air at 15atm. The molten sulphur is then continuously pumped out and allowed to solidify in a large tank. The sulphur obtained is 99.5% pure.
ALLOTROPES OF SULPHUR
The two main crystalline allotropes of sulphur are:
- Rhombic sulphur: A bright yellow octahedral crystalline solid. Each crystal is made up of S8 Rhombic sulphur is stable below 96oC.
- Monoclinic Sulphur: This is amber coloured solid sulphur consisting of needle-shaped S8 Stable above 96oC. It easily reverts to Rhombic below 96oC. The transition temperature between Rhombic and Monoclinic is 96oC.
Comparison of the Physical Properties of Rhombic and Monoclinic Sulphur
- Rhombic Sulphur melts at 113oC; while monoclinic sulphur melts at 119o
- Rhombic sulphur has a bright yellow colour; while monoclinic sulphur has an amber colour.
- Rhombic sulphur has an octahedral shape; while monoclinic sulphur is needle-like in shape.
- Rhombic sulphur is translucent; while monoclinic is transparent.
Sulphur also exists as a non – crystalline solid. These are
- Amorphous sulphur
- Plastic sulphur
EVALUATION
- Briefly explain the Frasch process.
- Name the two allotropes of sulphur and state their transition temperature.
PHYSICAL PROPERTIES
- Sulphur is a yellow solid.
- It is insoluble in water but soluble in toluene and carbon (IV) sulphide.
- It is a poor – conductor of heat and electricity.
- It melts at 119oC and boils at 444o
CHEMICAL PROPERTIES
- It reacts directly with metals to form sulphide (S2-)
Fe(s) + S(s) → FeS(s)
- It reacts with excess oxygen to form sulphur (IV) oxide
O2(g) + S(s) → SO2(g)
- It reacts with hydrogen to form hydrogen sulphide;
H2(g) + S(s) → H2S(g)
- It reacts with coke (carbon) to form carbon (IV) sulphide.
C(s) + 2S(s) → CS2(l)
USES
- Used in manufacturing tetraoxosulphate (IV) acid
- Used in the vulcanization of rubber
- Used as germicides
- Used in manufacturing bleaching agent
GENERAL EVALUATION/REVISION
- State THREE physical and chemical properties of sulphur.
- Outline THREE differences between monoclinic and rhombic sulphur.
- Descried the Frasch process for the extraction of sulphur.
- State TWO differences between a conductor and an electrolyte.
- List the steps involved in the treatment of water for municipal supply.
READING ASSIGNMENT
New School Chemistry for Senior Secondary Schools by O. Y. Ababio (6th edition), page 381-384
WEEKEND ASSIGNMENT
SECTION A: Write the correct option ONLY
- Sulphur is extracted by (a) Haber process (b) Frasch process (c) Solvay process (d) Contact process
- Which of the following is a crystalline allotrope of sulphur (a) Monoclinic (b) Plastic (c) Amorphous (d) Colloidal
- The density of rhombic sulphur is (a) 1.2 (b) 1.5 (c) 2.08 (d) 1.98
- The shape of monoclinic sulphur is (a) needle shape (b) hexagonal (c) octahedral (d) tetrahedral
- Sulphur is used for (a) making of cellulose (b) cooking rice (c) vulcanizing rubber (d) manufacturing glass
SECTION B
- Give three differences between rhombic sulphur and monoclinic sulphur
- Briefly describe the extraction of sulphur
We have come to the end of this class. We do hope you enjoyed the class?
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
In our next class, we will be learning about Compounds of Sulphur. We are very much eager to meet you there.

This is very good .I like it for students ♥️
it’s very helpful to users mostly the students, and it’s easy to understand.
good
I want to see the full note