Back to: CHEMISTRY SS2
Welcome to SS2!
We are eager to have you join us in class!!
In today’s class, We will be discussing The Periodic Trend. We hope you enjoy the class!
CONTENT
- Historical Development of the Periodic Table/Periodic Law.
- Features of the Periodic Table.
- Periodic Classification into Blocks and Families.
- Families of Elements.
THE PERIODIC TABLE
The periodic table is the table which shows the arrangement of elements in the order of increasing atomic number.
The modern periodic law states that the properties of elements are a periodic function of their atomic number.
FEATURES OF THE PERIODIC TABLE
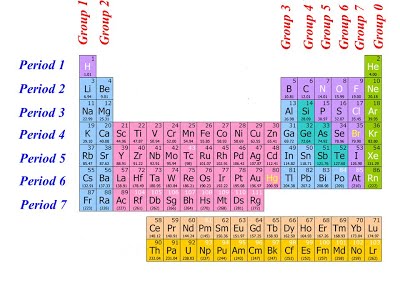
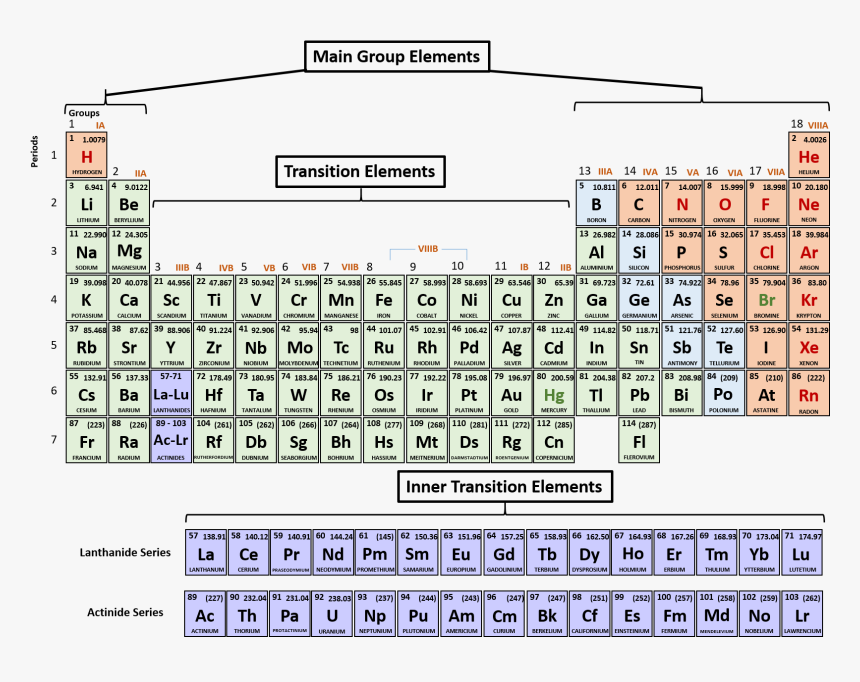
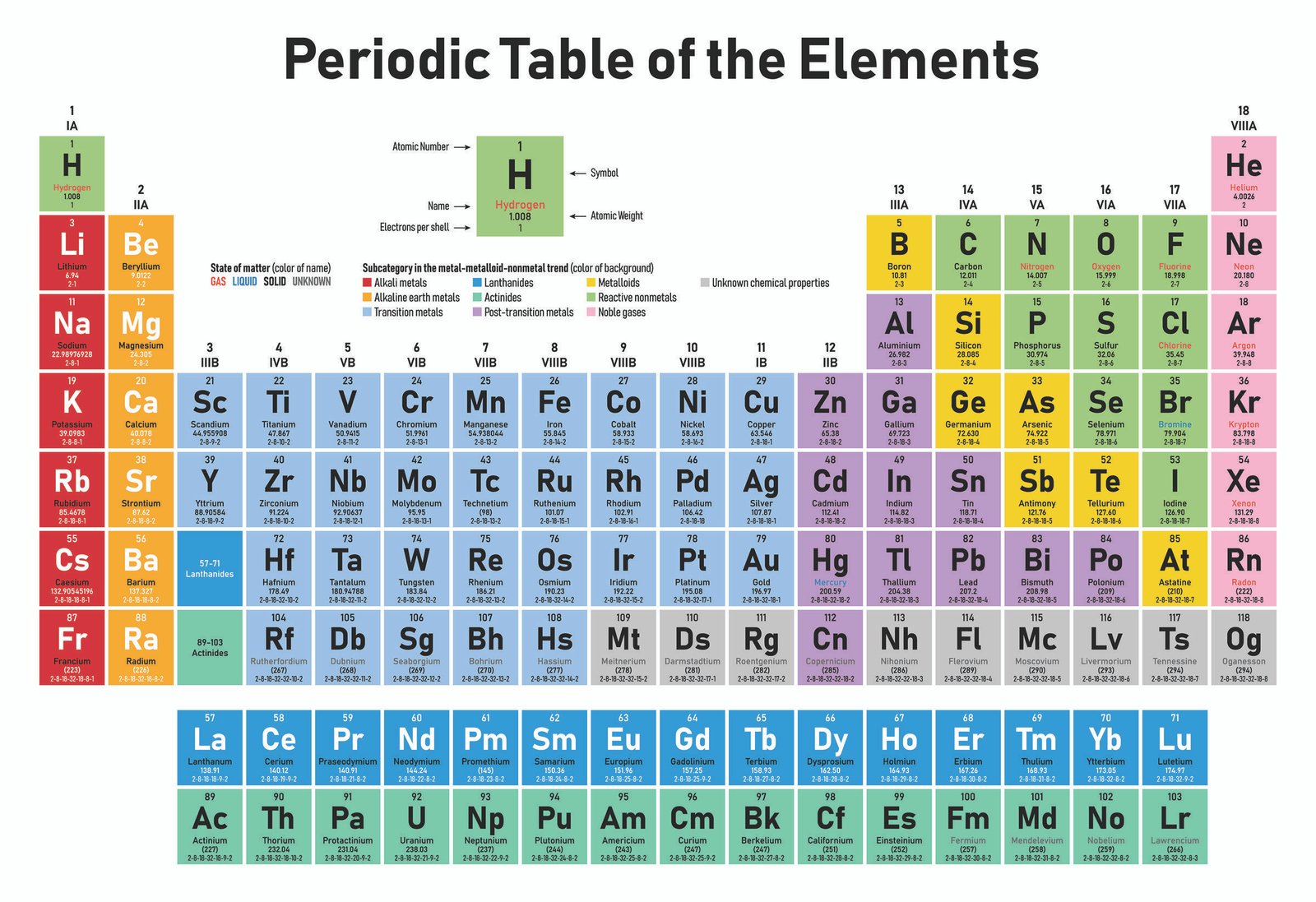
The modern form of the periodic table is divided into eight vertical columns known as GROUPS and seven horizontal rows known as PERIODS.
GROUPS:
The vertical columns of elements or groups are numbered from I to VIII (or 0). Elements in the same group have the same number of electrons in the valence shell. Hydrogen can be placed in group I or VII because it can donate its one electron like the group I elements or accepts electron like group VII elements. But for convenience and because of its simple valence electron, it is placed in group I. In group VIII, which is also group 0, Helium has two electrons while the other elements have eight valence electrons. Besides the eight groups, there are also, the transition groups of elements. These lay between group II and III in the periodic table.
PERIODS:
The horizontal rows of elements or periods are numbered from 1 to 7. Elements in the same period have the same number of electron shells. Among the elements in period six and seven are the elements of Lanthanides and Actinides series knows as inner transitions metals.
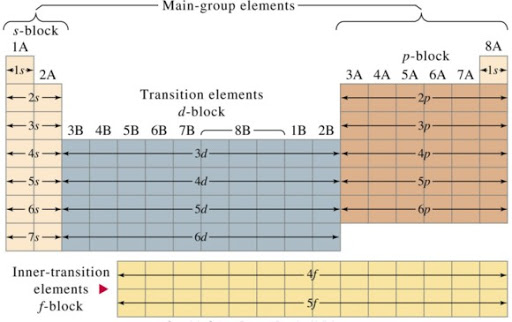
PERIODIC CLASSIFICATION INTO BLOCKS AND FAMILIES
The elements in the periodic table may be divided into blocks according to the orbital their valence electrons are found which is responsible for the positions of the elements. The s-block elements have s-electrons in the outermost energy level, while the p-block has both s and p-electrons. The transition elements contain d-electrons in addition to its s and p-electrons, while the lanthanides and actinides contain f-electrons in addition to the s, p and d electrons.
Element Atomic Number Electronic configuration.
H 1 1s1
He 2 1s2
Li 3 1s2 2s1
Be 4 1s2 2s2
B 5 1s2 2s2 2p1
C 6 1s2 2s2 2p2
N 7 1s2 2s2 2p3
O 8 1s2 2s2 2p4
F 9 1s2 2s2 2p5
Ne 10 1s2 2s2 2p6
Na 11 1s2 2s2 2p6 3s1
Mg 12 1s2 2s2 2p6 3s2
Al 13 1s2 2s2 2p6 3s2 3p1
Si 14 1s2 2s2 2p6 3s2 3p2
P 15 1s2 2s2 2p63s23p3
S 16 1s2 2s2 2p63s2 3p4
Cl 17 1s2 2s2 2p6 3s2 3p5
Ar 18 1s2 2s2 2p6 3s23p6
K 19 1s2 2s2 2p6 3s23p6 4s1
Ca 20 1s2 2s2 2p6 3s23p6 4s2
EVALUATION
- State the periodic law.
- Explain the basis on which elements are arranged in the periodic table.
FAMILIES OF ELEMENTS
Elements in the same group may be said to belong to a family since they show similar properties because their atoms have the same number of valence electrons. At the same time, certain properties of the element in the same group show a gradual change with an increase in atomic number. Such gradual change of property within a group is known as GROUP TREND.
GROUP I
The group I elements include Lithium (Li), Sodium (Na), Potassium(K), Rubidium(Rb), Caesium (Cs), and Francium (Fr). They are univalent elements. Their properties are as follows:
- They are good reducing agent since they can readily donate one electron to form cations.
- They are metals, thus they are good conductors of electricity and heat.
- They react vigorously with cold water to liberate hydrogen gas and form alkali, hence, they are known as ALKALI METALS. Example2Na(s) + 2H2O(l)→ 2NaOH(aq) + H2(g)
- The oxides of group I elements dissolve in water to give very strong alkalis. Example
K2O(s) + H2O(l)→ 2KOH(aq)
GROUP II
Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), and Radium (Ra) belong to group II. They are divalent elements and are also known as alkaline earth metals. Their properties include:
- They ionize by donating their two valence electrons; hence they are good reducing agents.
- They are hard metals, ductile malleable and can conduct both electricity and heat.
Beryllium does not react with cold water or steam, magnesium reacts with steam only while calcium reacts slowly with cold water to liberate hydrogen gas.
- Their oxides are insoluble in water except fort calcium oxide which dissolves in water to form an alkali.
CaO(s) + 2H2O(l)→Ca(OH)2(aq)
GROUP III
The group III elements are: Boron (B), Aluminum (Al), Gallium (Ga), Indium (In) and Thallium (Tl). They are trivalent elements. Their properties are:
- They are reducing in nature since they can donate their three electrons to form electrovalent compounds.
- Only aluminium can react with steam at about750oC to liberate hydrogen gas.
- Oxide and hydroxide of aluminum is amphoteric in nature, i.e, they have both acidic and basic properties. Example
Al2O3(s) + 3H2SO4(aq)→ Al2(SO4)2(aq) + 3H2O(l)
2Al(OH)3(s) + NaOH(aq)→NaAl(OH)4(aq)
GROUP IV
Group IV elements include Carbon (C), Silicon (Si), Germanium (Ge), tin (Sn) and lead (Pb). They form covalent compounds.
- They exhibit two oxidation states: +2 and +4. Due to the inert pair effect of electrons in the s-orbital of the valence shell, the +2 oxidation state becomes more prominent down the group.
- Electropositivity increases down the group. Carbon is a non-metal; silicon and germanium are metalloids while tin and lead are metals.
- Carbon does not react with water in any form, but silicon and tin react with steam at red heat to form +4 state oxides and hydrogen.
Si(s) + 2H2O(l)→ SiO2(s) + 2H2(g)
GROUP V
Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb) and Bismuth (Bi) belong to group V. They have the following properties
- They exhibit oxidation states of -3 and -5.
- They also show a group trend. Nitrogen and phosphorus are non-metals; arsenic and antimony are metalloids while bismuth is a metal.
- They are electron acceptors, hence they are oxidizing in nature.
- They form oxides that dissolve in water to form acids except for nitrogen (I) oxide.
GROUP VI
Elements in group VI include Oxygen (O), Sulphur (S), Selenium (Se), Tellurium (Te), and Polonium (Po). Their properties are as follows:
- They are non-metals and exist as solids at room temperature except for oxygen
- They are electron acceptors and oxidizing in nature.
- They do not react with water in any form. But oxygen and sulphur combine directly with hydrogen to yield water and hydrogen sulphide respectively.
GROUP VII
Elements in this group include Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I) and Astatine (At). They are known as halogens (salt-makers). Their properties include:
- They ionize to form univalent anions.
- They exist as diatomic molecules.
- As electron acceptor, all halogens are good oxidizing agents.
- They exhibit the following group trend. Fluorine and chlorine are gases, bromine is a liquid and iodine and astatine are solids at room temperature.
GROUP VIII (0)
The elements in group 0 are known as rare or noble gases because they are non-reactive and exist freely as monoatomic molecules in the atmosphere. The elements that belong to this group are: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Rn).
TRANSITION ELEMENTS
These are elements found in-between group II and III of the periodic table. The first transition series consists of elements: Scandium (Sc), Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel (Ni), Copper (Cu) and Zinc (Zn). Transition elements have the following properties:
- High tensile strength
- High melting and boiling points
- Variable oxidation states
- Formation of coloured ions
- Formation of complex ions
- Paramagnetic in mixture
- Catalytic ability
LANTHANIDES (RARE EARTH ELEMENTS):
These are found in period six. This series begins with Lanthanum (La) and ends with Lutetium (Lu). They are altogether 15 and resemble one another greatly.
ACTINIDES AND THE ARTIFICIAL ELEMENTS:
The actinides are similar to the Lanthanides. They are found in the seventh period, which starts with Actinium (Ac) and ends with Lawrencium, (Lr). The famous Uranium is in this group.
The elements with atomic numbers from 93 to 103 are known as artificial elements. This is because they do not occur naturally but were formed during nuclear reactions.
GENERAL SELF EVALUATION/REVISION
- State the division of the modern periodic table
- Hydrogen can be placed in group I or VII. Explain
- Write the electronic configuration of the following element and state the group and period to which they belong: 6C, 11Na, 14S, 18Ar
- State the number of unpaired electron in each of the following atom/ions: 12Mg2+, 16S2-17Cl
- Name ten laboratory apparatuses and state their uses
READING ASSIGNMENT: New School Chemistry for Senior Secondary School by O.Y. Ababio, pages 141-143, 150-154
We have come to the end of this class. We do hope you enjoyed the class?
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
In our next class, we will be talking about The Periodic Trend. We are very much eager to meet you there.

You guys are doing a great job with classnotes.ng, a perfect solution to the problem of accessing quality education in this period. Will highly recommend.
What an interesting class
yes
WOW! I really enjoy the class
Very well it is better
Do you have these tables in pdf form so I can print them out to study?
It would be well appreciated
Thanks
I really enjoyed the lesson
I really enjoyed it
I came for a little thing and got more than I wanted
Great job well done
I really enjoyed the class thanks
very interesting and enthusiasing
How do I solve electronic configuration
please can it be possible for student to submit the week assignment on the website for assessments
very simple and understanding….thank you
very very far good tanx
You re doing a great job. I just love this website
Glad you found it helpful😊 For even more class notes, engaging videos, and homework assistance, just download our Mobile App at https://play.google.com/store/apps/details?id=com.afrilearn. It’s packed with resources to help you succeed🌟
it made my note better to understand.
I love it
We’re glad you found it helpful😊 For even more class notes, engaging videos, and homework assistance, just download our Mobile App at https://play.google.com/store/apps/details?id=com.afrilearn. It’s packed with resources to help you succeed🌟
chemistry is made simple with this class
I love it
Wonderful
I may not be in your set, but I’m not ashamed to say it got to a certain point I didn’t understand anymore🥴. But all, in all I loved the class ❤️
Hi Ore, we sympathize with you on the challenge you are facing. Kindly explain what you don’t understand so we can help you further. Thank you
i understand it very well, thank you
interesting topic
Nice teaching
The class was very interesting, I loved it
I love this note, this is just exactly what i wanted
Nice teching
The note is well detailed and so easy to comprehend, well done