Back to: CHEMISTRY SS2
Welcome to Class !!
We are eager to have you join us !!
In today’s Biology class, We will be learning about Periodic Trends. We hope you enjoy the class!
CONTENT
- The Periodic Properties of Elements: Atomic size, Ionic size, Ionization Energy, Electron Affinity, Electronegativity down the group and across the period.
- Diagonal Relationship in the Properties of Elements in the Periodic Table.
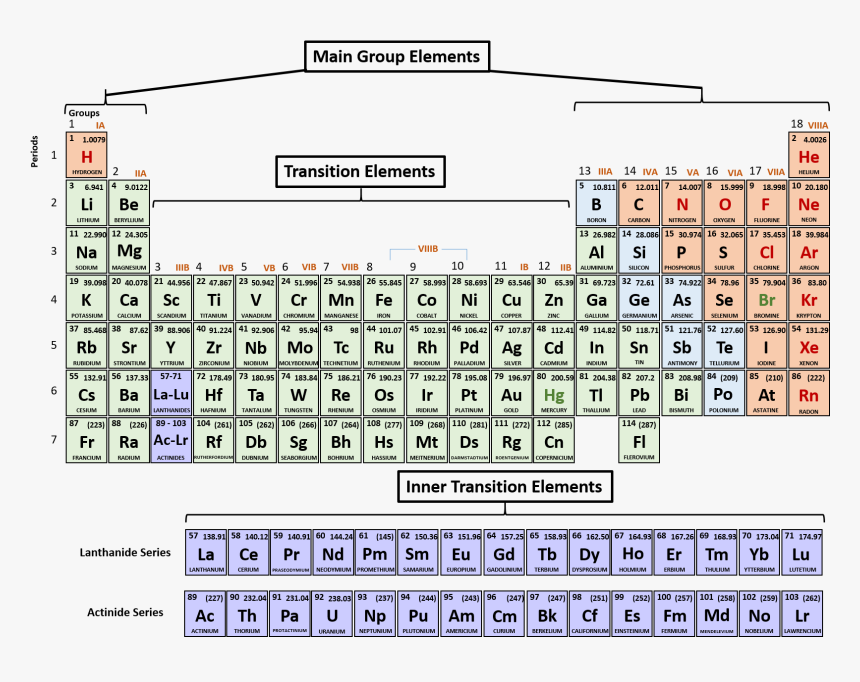
PERIODICITY
Periodicity with respect to the periodic table is defined as the variation in the properties of elements in a regular pattern both down the groups and across the periods.
REASONS FOR PERIODIC VARIATION IN PROPERTIES
Two opposing factors are responsible for the variation in the properties of elements. These are
- Effect of increasing positive nuclear charge.
- Screening effect of inner-shell electrons.
EFFECT OF NUCLEAR CHARGE AND INNER SHELL ELECTRONS
- Nuclear charge exerts a force of attraction on the electrons towards the nucleus. This force of attraction increases as a result of increasing nuclear charge across periods.
- Inner shell electrons screen the nuclear charge from exerting its force of attraction outwards to outer electrons. Screening effect is constant across each period but increase down the groups.
The overall effects of these are:
- Effect of increasing nuclear charge overpowers the screening effect of inner shell electrons across a period.
- Screening effect of inner shell electrons overpowers the effect of increasing nuclear charge down the groups.
These effects are the reasons for periodic variations in the properties of elements.
ATOMIC PHYSICAL PROPERTIES OF ELEMENTS THAT SHOW PERIODIC VARIATION
- ATOMIC RADIUS: Atomic radius is one half the distances between two covalently bonded atoms. For electrovalent compounds, the ionic radius is a measure of the distance between the centre of the ion and the centre of its nearest neighbour of opposite charge.
- IONIZATION ENERGY: Ionization energy is the energy required to remove one mole of electrons from one mole of a gaseous atom to produce one mole of gaseous ions.
- ELECTRONEGATIVITY: Electronegativity is the power of an atom of an element to attract electrons to it to become negatively charged.
- ELECTROPOSITIVITY: Electropositivity is the power of the atom of an element to lose an electron and become positively charged.
- ELECTRON AFFINITY: Electron affinity is the energy change which accompanies the addition of one mole of electrons to one mole of a gaseous atom of an element to form negatively charged ions.
EVALUATION
- State five atomic physical properties of elements.
- Define two of the stated atomic physical properties
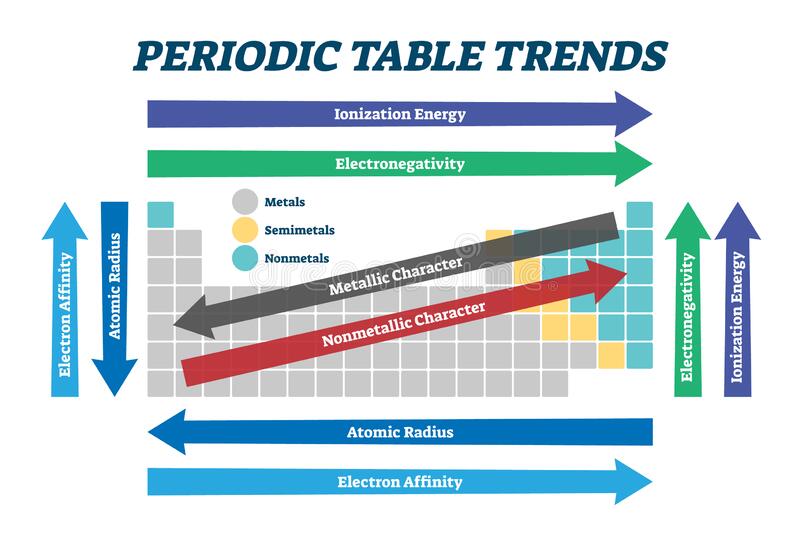
TRENDS IN ATOMIC PROPERTIES
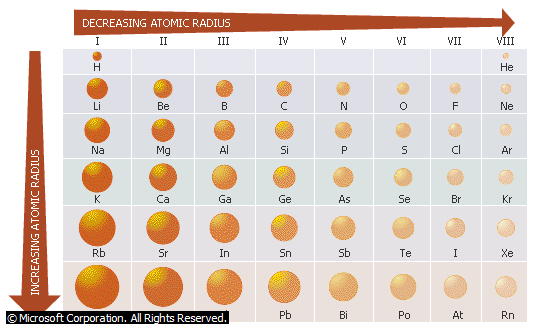
- Atomic radius decreases across the periods from left to right while it increases down the groups from top to bottom.
- Ionization energy increases across the periods left to right while it decreases down the groups from top to the bottom.
- Electronegativity increases from left to right across each period while it decreases down the groups from top to bottom.
- Electropositivity decreases from left to right across the period while it increases down the groups from top to bottom.
- Electron affinity decreases from left across each period while it increases down the groups.
- Ionic radius decreases for metal across the periods and increases for non – metals.
Note: Ionic radius generally increases down the groups and decreases across the periods.
NOTE
Cations formed by electron loss are smaller in size than the neutral atom from which they are formed and anions formed by electron gain are larger in size than the neutral atoms that form them. Also, the larger the charge on a cation, the smaller the ionic radius and the larger the charge on the anion, the larger the ionic radius.
EVALUATION
State the trends of the following across the periods and down the groups
- Ionization energy
- Atomic radius.
VARIATION IN OTHER PHYSICAL PROPERTIES
- MELTING POINT AND BOILING POINT:
The melting point is the temperature at which a solid begins to melt at a particular pressure. The boiling point is the temperature at which a liquid begins to boil at a particular pressure.
The melting and boiling points of elements increase from groups I to III across each period with group IV elements having the highest value and decrease from group V to 0. The melting and boiling point decrease down the groups from group I to IV and increase down the groups from group V to 0. To account for this trend, in the properties across period, there is an increase in metallic bond from group I to III and a lot of energy is needed to break this bond. The presence of giant covalent lattice in group IV elements results in the high melting and boiling points. From group V to 0, there is decrease in the weak intermolecular force of attraction among the molecules of the atoms of elements. Thus, there is a decrease in melting and boiling point.
- ELECTRICAL AND THERMAL CONDUCTIVITY: These properties of elements decrease across the period and increase down the group. Thus, metals are good electrical and thermal conductors while non- metals are poor electrical and thermal conductors.
EVALUATION
- Explain variation of ionic radius in the periodic table.
- Explain the variation of melting and boiling points in the periodic table.
VARIATION IN CHEMICAL PROPERTIES
The chemical properties of elements also exhibit periodic variation. This is also shown in the properties of the compounds of the elements.
GROUPS: Elements in the same group have similar chemical properties because they have the same number of valence electrons. The four groups of elements, which show great similarity in their chemical properties, are
- Group 1 element or the Alkali metals.
- Group II elements or the Alkaline earth metal
- Group VII elements or the Halogens
- Group 0 elements or the noble or rear gases
PERIOD: Elements in the same period do not exhibit similar chemical properties. Chemical properties change across the periods.
The following chemical properties shall be considered
- Chemical reactivity
- Compound thermal stability
- Ease of formation of ions
- Metallic properties and Non – metallic properties
- Chemical reactivity decrease from group I – III for metals and increase from group IV to VII for non-metals across the periods while it increase down groups for metal and decreases down groups for non-metals.
- Compound thermal stability decrease from Group I to III for metals and increases from IV to VII for non- metals across the period while it increases down each group for metals and decreases for non-metal respectively.
- Ease of formation of ions decreases from Group I – III across the periods for metals and increases from IV to VII for non-metals while it increases down the group for metals and decreases down the groups for non-metals
- Metallic property decreases across the periods and increases down groups while Nonmetallic property increases across periods and decreases down groups.
EVALUATION
- State the valence electron of each of the group of elements:
- Alkali metals (b) Halogens (c) Alkaline earth metals
- Briefly explain the variation in the following chemical properties:
(a) Chemical reactivity (b) Ease of formation of ions (c) Metallic property
DIAGONAL RELATIONSHIP IN THE PROPERTIES OF ELEMENTS IN THE PERIODIC TABLE
Diagonal relationship is said to exist between certain pairs of diagonally adjacent elements in the second and third periods of the periodic table example Lithium and Magnesium, Beryllium and Aluminum, Boron and Silicon.
It is found that the chemistry of a first-row (second period) element often has similarities to the chemistry of the second-row (third period) element being one column to the right of it in the periodic table. Thus, the chemistry of Lithium has similarities to that of Magnesium, the chemistry of Beryllium has similarities to that of Aluminium, and the chemistry of Boron has similarities to that of Silicon. These are called diagonal relationships. (It is not as noticeable after Boron and Silicon.) The reasons for the existence of diagonal relationships are not fully understood, but charge density is a factor. For example, Li+ is a small cation with a +1 charge and Mg2+ is somewhat larger with a +2 charge, so the charge density on each ion is roughly the same. Let’s consider the Li–Mg pair: (under room temperature and pressure)
- Lithium and Magnesium form only normal oxides whereas sodium forms peroxide and metals below sodium, in addition, form superoxides.
- Lithium is the only Group I element which forms a stable nitride, Li3Magnesium, as well as other Group II elements, also forms nitrides.
- Lithium trioxocarbonate (IV) and Lithium fluoride are sparingly soluble in water. The corresponding Group II salts are insoluble.
- Chlorides of both Li and Mg are deliquescent (absorb moisture from surroundings) and are soluble in ethanol. Also, Lithium chloride, like magnesium chloride (MgCl2.6H2O) separates out from solutions as hydrated crystal LiCl.2H2
GENERAL EVALUATION/REVISION
- Define the following terms: Ionization energy, Electronegativity, Electron affinity
- Describe the variation in each of the terms defined in (1) above across a period and down a group
- Arrange the following ions in order of increasing size: Na+, Ca2+ and Al3+. Give reason for your answer.
- Define physical and chemical change giving two examples each.
- State three differences between physical and chemical changes.

can i define electro positivity as the ability of power of an atom of an element to lose of give out electron to become gaseous postively charged
Show me the families of element