Back to: CHEMISTRY SS3
Welcome to class!
In today’s class, we will be talking about amines and amides. Enjoy the class!
Amines and Amides
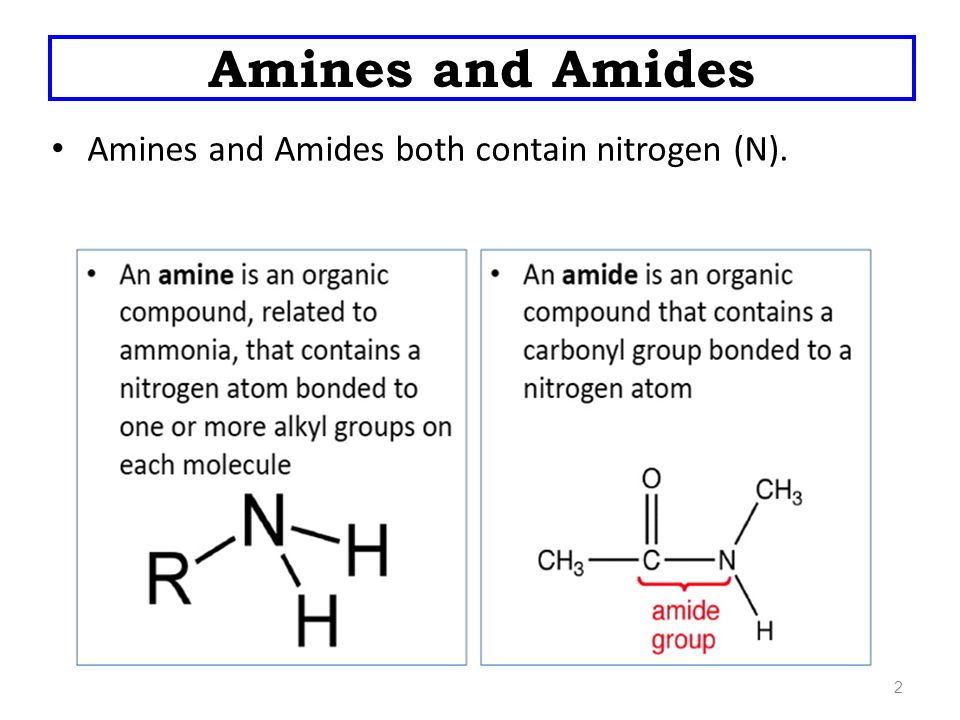
Amines
It has a functional group of NH2.
Preparation
They are derivatives of ammonia where one or more hydrogen atoms have been replaced by alkyl or aryl groups e.g. RNHz, R2NH.
Classification
Amines can be classified according to an alkyl group.
- Primary amine with one alkyl group e.g. RNH2 or
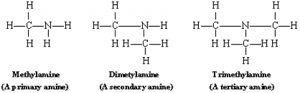
- Secondary amine with 2 alkyl groups e.g. R2NH or
- Tertiary amine with 3 alkyl groups e.g. R3N or
Physical properties
- They can dissolve in water.
- They are gases and liquid.
- They have a fishy odour.
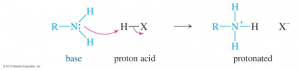
Chemical properties
- As bases, they neutralize acids.
- They dissociate/ionize in water e.g. CH3NH2 + H2O CH3NH3++ OH–
Uses
- Used in making nylon
- They can also be used in making polyamide.
Evaluation
- State two (2) physical properties and two (2) chemical properties of amine.
- Give the classes of amine according to the number of their alkyl groups.
Amides
It has a functional group of –C which is known as the carbonamide group.
NH2
Structure
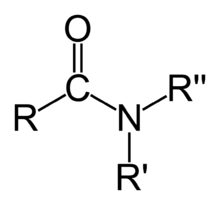
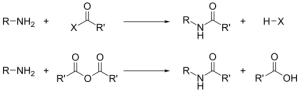
Preparation
- Amide e.g. ethanmide can be derived from ethanoic acid in the presence of ammonia.
- Amide is commonly prepared by reacting esters with concentrated aqueous ammonia.
E.g. CH3COOC2H5 + NH3 → Ethanamide CH3CONH2 + C2H5OH
- They can also be prepared by removing a molecule of water from ammonium salt of carboxylic x acids.
e.g. CH3COONH4 → CH3CONH2 + H2O
Physical properties
- The only methanamide is a liquid while others are solid.
- They have a high melting point and high boiling point.
Chemical properties
- Amide can be hydrolysed in the presence of alkali and mineral acid e.g.
CH3CONH2 + H2O → CH3COOH + NH3
- In the presence of sodium hydroxide and bromine, amides produce amines with the elimination of one carbonyl group.
e.g. CH3CONH2 + Br2 + 4NaOH → CH3NH2 + 2NaBr + Na2CO3 + 2H2O
Uses
- Used in the preparation of amines.
- Used in making synthetic resins and plastics.
- It can also be used in making fertilizer.
Carbamide/urea
This is an amide of hydrogen trioxo carbonate (IV) acid. It is produced by compressing CO2 and NH3 at high pressure at 2000C.
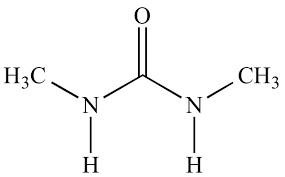
N.B.: Urea is produced in our body and excreted in the urine.
General evaluation
- Write the structural formula of amide.
- Give one different between amine and amide.
Reading assignment
New School Chemistry by O.Y. Ababio pages 520-521
Weekend assignment
- The tertiary amine is represented as follow A. R2NH2 B. (CH3)2NH C.R2NH3 D. R2N
- Which of the following has fishy odour A. alkanoic acid B. alkanol C. amide D. amine
- Amide can be regarded as derivatives of ………… A. alkanol B. polycarboxylic acid C. monocarboxylic acid D. carboxamide group
4.During the hydrolysis of amides, one of the following is produced. A. monocarboxylic acids B. water C. H2SO4 D. NaOH
- Carbamide is an example of A. amine B. alkane C. alkyl D. amide
Theory
1(a). State two (2) physical properties of amide.
(b). How would you prepare ethanamide from an ester?
2(a). State two (2) chemical properties of amide.
(b). How would you identify an example of amine in the laboratory?
We hope you enjoyed the class.
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.

Wonderful