Back to: CHEMISTRY SS3
Welcome to class!
In today’s class, we will be talking about Natural and synthetic polymers. Enjoy the class!
Natural and Synthetic Polymers
A polymer is a final product, macromolecule of high molecular mars. It consists of repeating units and its general molecular formula may be represented as [repeating units]n where n is a very large whole number.
N.B:- All polymers are macromolecules, but not all macromolecule but it is not polymeric.
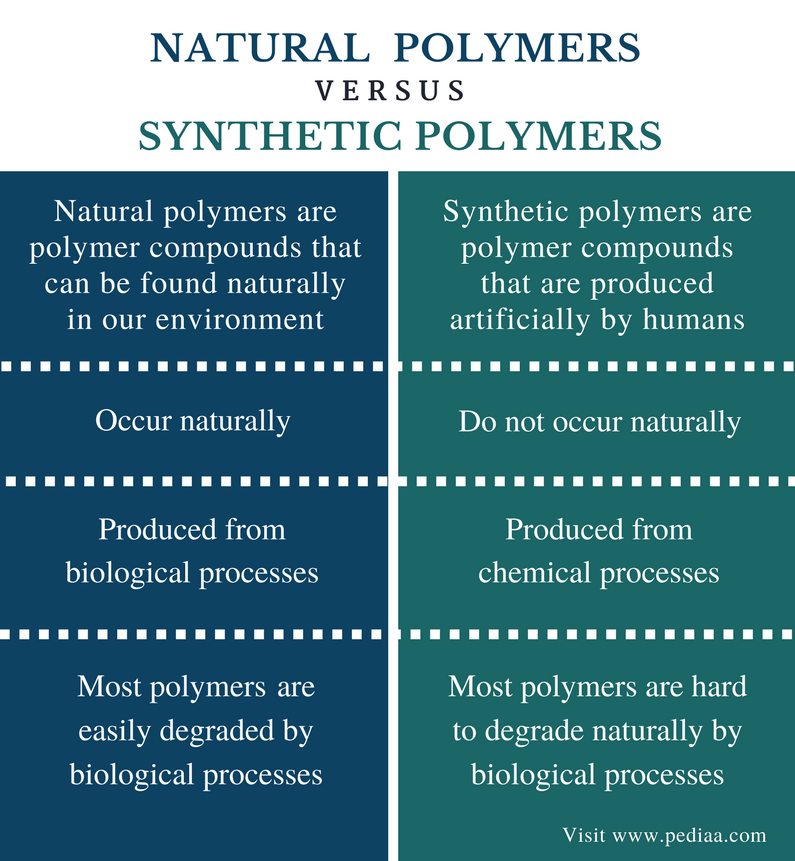
Natural polymers:
These are organic compounds which can be found in living thing e.g. carbohydrates like starch and cellulose and all proteins fats and oils are not large enough to be grouped as giant molecule or polymer.
Synthetic polymers:
These are called plastics e.g. nylon polythene etc.
Evaluation
- State a difference between natural polymers and synthetic polymer with an example each.
- What is another name for synthetic polymer?
Polymerization
This is the process whereby two or more monomers link/joins together to form a compound of high molecular mass.
Types of polymerization
- Addition polymerization: these occur when two or more of the same monomers join together to form the polymer without elimination of any small molecules.
Characteristics of a monomer
- It must be simple.
- Unsaturated
- There should be double bonds between the carbon atoms.
e.g. n[CH2CH2 ] ……..CH2CH2[CH2CH2]nCH2CH2……
- Condensation polymerization: This is a process whereby two or more smaller molecule (monomers) join together to form a giant molecule (polymer) with the elimination of trace/small molecule such as waters ammonia, hydrogen chloride.
Types of condensation polymerization
- Copolymer: This is formed from two condensing monomers of different types.
- Homopolymer: It is formed from monomers of the same type.
Conditions necessary for polymerization
- High temperature
- High pressure
- Presence of catalyst (initiator) e.g. oxygen, hydrogen peroxide.
Evaluation
- State two conditions necessary for polymerization of ethane to from polythene.
- Mention one difference between additional polymerization and condensation polymensation.
Plastics
Plastics are synthetic which can be heated or pressured to form any shape.
Thermoplastic:
Thermoplastics are a type of synthetic materials which can be heated and remoulded to any shape e.g. nylon, polythene, polypropene, Perspex etc.
Thermosets:
Thermosets, on the other hands, cannot be softened or melted by heat and remoulded once they are formed e.g. urea-methanal, bakelite.
Thermoplastics and thermosets
| Thermoplastics | Thermosets |
| Polythene | Bakelite |
| Polypropene | Urea-methanal |
| Polystyrene | |
| Nylon | |
| Terylene | |
| Perspex |
Resins
This is obtained from the rubber tree. The fluid obtained from the tree can be heated and changed to an elastic solid known as rubber. The rubber consists of 2-methyl but-1, 3- diene monomers known as isoprene.
Vulcanization:
This is the process of heating natural rubber with sulphur to give the rubber a greater tensile, strength, durability and elasticity over a wide range of temperature.
Synthetic rubber:
Examples of synthetic rubbers are poly 2-chlorobuta -1,3diene, styrene-butadiene rubber (SBR), poly bute-1,3-diene and poly 2-methyl propene.
General evaluation
- What is resin?
- State two (2) differences between thermoplastic and thermoset.
Reading assignment
New School Chemistry by O.Y. Ababio pages 523-531
Weekend assignment
- The following are examples of small molecules based during the polymerization process. A. acid B. HCl C.H2O D. NH3
- Polymerization of ethane produces …………… A. Perspex B. isoprene C. polythene D. ammonia
- Bakelite is a good example of ………….. A. natural rubber B. thermoset C. thermoplastic D. additional polymerization
- Starch and cellulose are good examples of ………… A. polythene B. natural polymer C. synthetic polymer D. food
- Joining together of smaller molecules to form a giant molecule is called ……….. process. A. hydrogenation B. saponification C. esterification D. polymerization
Theory
1(a) What are the conditions necessary for polymerization.
(b) List two types of polymerization.
2 (a) Write an equation for the preparation of polythene from ethane.
(b) What is the monomer present in the following:
- polythene
- polyvinyl/chloride
- polytetrafluoro ethane
- polypropene
In our next class, we will be talking about Carbohydrates. We hope you enjoyed the class.
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
