Back to: CHEMISTRY SS3
Welcome to class!
In today’s class, we will be talking about unsaturated hydrocarbons – alkynes. Enjoy the class!
Unsaturated Hydrocarbons – Alkynes

Alkynes are the homologous series of unsaturated hydrocarbon with a general molecular formula CnH2n-2.
Alkynes show a high degree of unsaturation than alkenes, hence, they are chemically more reactive than the corresponding alkenes or alkanes.
Examples are:
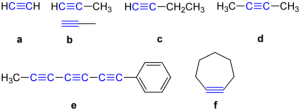
Ethyne
Ethyne is the first member of the alkyne series. It has a molecular formula, C2H2, and a structural formula of HC = CH.
Laboratory preparation
Ethyne is usually prepared in the laboratory by the action of cold water on calcium carbide. The reaction is carried out on a heap of sand to prevent the flask from cracking as a result of the large quantity of heat evolved.
Evaluation
- Write and name all possible structure of hexyne
- How can you prepare a few jars of ethyne in the laboratory?
Nomenclature
The naming of the alkyne is obtained by substituting “ane” in alkanes with ‘ene’.
Physical properties
- Ethyne is a colourless gas with a characteristic sweet smell when pure.
- It is only sparingly soluble in water
- It is slightly less dense than air.
- It is unstable and may explode on compression to liquid.
Chemical properties
- Combustion: it undergoes combustion reaction in air to form water and carbon(iv) oxide
2C2H2 + 5O2 → 2H2O + 4CO2.
NB: In limited air, it burns with very smoky and luminous flame because of its high carbon content. But in plenty of air and appropriate proportion, it burns with the non-luminous very hot flame of about 3000oC.
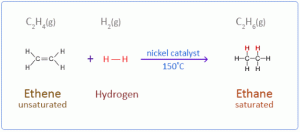
- Additional Reaction: Ethyne undergoes addition reaction to produce an unsaturated product with double bonds and then a saturated compound with a single bond.
a. With hydrogen in the presence of nickel as a catalyst.
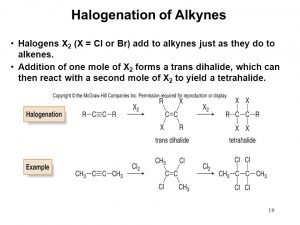
b. Halogenation: e.g Cl2, Br2, I2
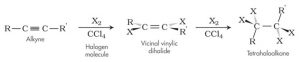
c. Addition of Halides:
Hydrogen halide reacts with ethyne to produce halo-alkene and further halogenation produce halo-alkane.
E.g.
- Addition reaction with water through dilute tetraoxosulphate (vi) acid in the presence of CuSO4 as the catalyst to form ethanol.
- Addition reaction with alkaline KMnO4 added to ethyne, it first turns to the green from purple and then to colourless.
- Polymerization:
In the presence of complex organic –nickel as the catalyst to produce benzene.
3 C2H2 → C6H6
3 (H – C = C – H ) → C6H6
- Substitutional Reaction
- With ammomiacal solution of copper (1) chloride to form reddish brown copper (I) dicarbide
C2H2 + 2CuCl → Cu2C2 + 2HCl
H – C = C – H + 2CuCl → Cu – C = C- Cu + 2HCl
- With ammomiacal silver trioxonitrate (v) to form white silver dicarbide
C2H2 + 2AgNO3 → Ag2C2 + 2HNO3.
H- C = C – H + 2AgNO3 → Ag – C = C – Ag + 2HNO3.
N.B: Alkynes can be distinguished from an alkene by reacting with ammonical metals of copper(I) chloride and silver trioxonitrate (vi).
Uses
- It is used to produce an oxyacetylene flame for cutting and welding of metals
- Used in the manufacture of PVC plastics
- It is used in miner’s lamp
- Used in making synthetic fibre
- It is also used in making artificial rubber
Test for unsaturation
Unsaturated compound decolorizes bromine water.
General evaluation
- Give a chemical test to distinguish between alkyne and alkene.
- Describe a test for unsaturated compounds
Reading assignment
New School Chemistry by Y. O Osei yaw Ababio Page
Weekend assignment
- The concentration of hydrogen ion in a neutral solution is (a) 10-6 moldm-3 (b) 10-7moldm-3 (c) 5 x 10-7 moldm-3 (d) 1 x 10-8mol dm-3
- Hydrogen can be prepared in a large scale using the (a) Harber’s process (b) Down’s process (c ) Bosh Process (d) Contact Process.
- Which of the following hydrocarbons is alkyne? (a ) C2H4 (b) C2H6 (c) C2H2 (d) C3H8
- The final product of a complete reaction between ethyne and hydrogen gas is (a) ethane (b)methyl ethane ( c) ethane ( d) hydroethyne
- Ethyne polymerizes in the presence of organonickel complex as catalyst to form (a) polythene (b) benzene (c) polythene (d) methylbenzene.
Theory
- a. Calculate the H+ of a solution whose PH is 5.
- State three (3) uses of ethyne
- a. With the aid of labelled diagram, describe the laboratory preparation of ethyne. b. Give a chemical test to distinguish between ethane and ethyne.
In our next class, we will be talking about Aromatic Hydrocarbon. We hope you enjoyed the class.
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.

I don’t understand anything
How can we help you understand the lesson better?