Back to: CHEMISTRY SS3
Welcome to class!
In today’s class, we will be talking about metallic bonding. Enjoy the class!
Metallic Bonding
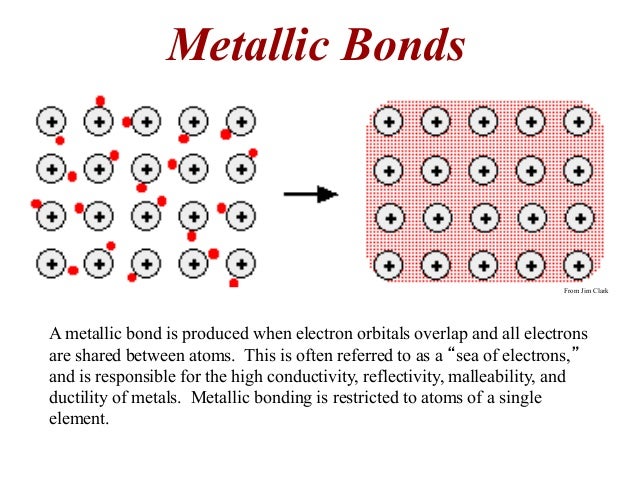
The atoms of metals are held together in the crystal lattice by metallic bonds.
Properties
- They are good conductors of electricity and heat
- High melting and boiling points
- They are malleable and ductile
- They ionize by losing electrons
Factors affecting the formation of metal strength
- The valence electrons
- Intermolecular bonding:
This is the type of chemical bonding which can be found in some molecular solid. Examples of intermolecular forces are
- Van der walls forces
- Hydrogen force/bond
Van der Waal forces: this is the weak attractive forces that exist between the molecule.
Importance of Van der Waal forces
- it is important in the liquefaction of gases
- it is used in the formation of molecular lattices like iodine and naphthalene crystals.
Evaluation
- State the properties of the following bonds. a. metallic bond b. Van der Waal
- Give the diagram of a named metallic element (bonding diagram)
Hydrogen bonding
Hydrogen bonding occurs when hydrogen is covalently bonded with strongly electronegative element e.g nitrogen, fluorine, oxygen.
These electronegative elements pall the shared pair of electrons in the covalent bonds toward themselves. Thus it results in dipole where the hydrogen is positive and the electronegative element is negative.
An electrostatic attraction set up when the positive pole of one molecule attracts the negative pole of another molecule.
NB: The attractive force that exists between the two poles is called a hydrogen bond.
Example of hydrogen bond
- Hydrogen fluoride molecules
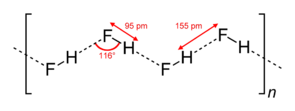
- Water molecules (ice crystal)

NB: There is a covalent bond in a molecule of water while hydrogen bond is formed in molecules of water.
Use
It helps in the formation of water, alkanols and some organic acid molecules
General evaluation
- Use a diagram to differentiate between a molecule of water and molecules of water
- State the molecules of a substance with the strongest hydrogen bond.
- Explain the simple cubic structure
Reading assignment
New School Chemistry by O.Y Ababio pg 294-298
Weekend assignment
- An example of intermolecular bonding is (a) Vander wall (b) metallic (c) ionic (d) covalent
- Both metallic substance and electrovalent compound are similar because (a) Both dissolve readily in water. (b) they have a low melting point. (c) they can conduct electricity. (d) they have a low boiling point.
- The dotted line in an intermolecular bond stands for ____ (a) oxygen (b) hydrogen bond (c) ionic bond (d) covalent bond
- Vander Wall force of attraction can be grouped as (a) Strongest force (b) hydrogen bond (c) weakest force (d) ionic bond.
- The metallic bond can easily be identified by the presence of (a) positive charge (b) negative charge (c) neutral charge (d) double bonds.
Theory
1. State the type of chemical bond found in the following substances
- Magnesium
- Sodium chloride
- Ammonium chloride
- Molecules of hydrogen fluoride
- A molecule of hydrogen
2. State four properties of the following chemical bonds:
- Electrovalent bond
- covalent bond
- dative bond
- hydrogen bond
- metallic bond
In our next class, we will be talking about Metals and their Compound. We hope you enjoyed the class.
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
