Back to: CHEMISTRY SS3
Welcome to class!
In today’s class, we will be talking about alkanoic acids. Enjoy the class!
Alkanoic Acids
Sources
The alkanoic acid or carboxylic acids are also called fatty acids because some of them are found in natural fats and oils. They contain the functional group called carboxyl group.
Nomenclature
The IUPAC name of each homologue is obtained by changing the “-e” ending of the corresponding alkane to “-oic” acid e.g. methanoic, ethanoic etc.
Structure
Alkanoic acid has a general molecular formula of CnH2n + 1COOH where n > 0. or RCOOH. Thus it has the following structure.
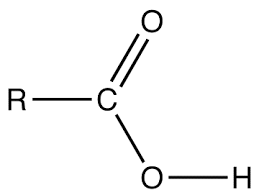
E.g. Ethanoic acid CH3COOH
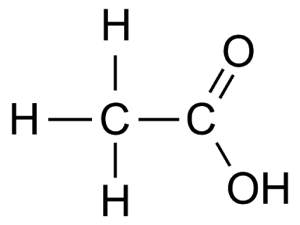
Preparation e.g. Ethanoic acid
Ethanoic acid can be prepared by the complete oxidation of ethanol by acidified sodium heptaoxodichromate (VI) solution. The oxidation reaction is two stages of reaction
- Ethanol oxidized to ethanol; CH3CH2OH O3 CH3CHO
- Ethanol oxidized to ethanoic acid; CH3CHO O3 CH3COOH
Or
CH3CH2OH + [O] CH3CHO + [O] CH3COOH
Physical properties
- It is a colourless liquid with a sharp and pungent smell.
- It has a sour taste.
- It is soluble in water.
- It freezes into ice-like at a temperature below 170C, therefore, called glacial ethanoic acid (anhydrous ethanoic acid).
- It has a boiling point of 1180C
- It turns blue litmus papers to red.
Chemical properties
- It libratescarbon(IV) oxide from either trioxocarbonate (IV) or hydrogen trioxocarbonate (IV) salts. 2CH3COOH + Na2CO3 → 2CH3CONa + H2O + CO2.
- It librates hydrogen gas when it reacts with highly electropositive metals e.g. Mg &Ca; 2CH3COOH + Ca → (CH3COO)2Ca + H2.
- As an acid, it neutralizes boxes or alkalis to form salts known as ethanoate and water only CH3COOH + NaOH → CH3COONa + H2O.
- It reacts with alkanols to form ester e.g. CH3COOH + CH3CH2OH → CH3COOCH2CH3 + H2O
- Reduction:
It can be reduced to ethanol in the presence of lithium tetrahydro aluminate III as a reducing agent (LiAlH4)
CH3COOH + 4H → CH3CH2OH + H2O
- It reacts with chlorine successively to form chloroethanoic acid e.g.
CH3COOH + Cl2 → CH2ClCOOH + HCl
CH2ClCOOH + Cl2 → CHCl2 COOH + HCl
CHCl2 COOH + Cl2 → CCl3COOH + HCl
Evaluation
- (a) State four (4) chemical properties of ethanoic acid.
(b) Give two (2) physical properties of ethanoic acid.
- How would you prepare ethanoic acid in the laboratory?
Classification of alkanoic acid
Alkanoic acids are classified based on the number of carboxylic groups present per molecules.
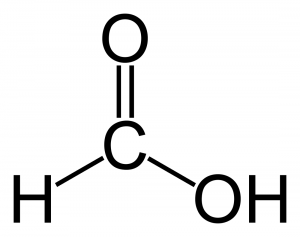
- Monocarboxylic Acids: these have one carboxylic group per molecule e.g. methanoic acid (HCOOH)
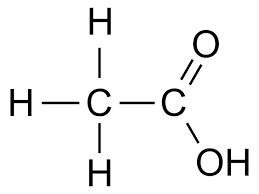
Ethanoic acid CH3 COOH
2. Dicarboxylic Acids: these have two carboxyl groups per molecules e.g. ethan -1, 2-dioe acid
(oxalic acid)
COOH
or
COOH
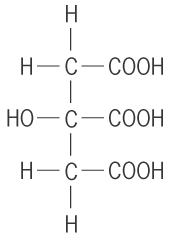
- Tricarboxylic acids: these have 3 carboxylic acids per molecule e.g. 2-hydroxy propan 1,2, 3-tricaboxylic acid.
N.B: Two important aromatic carboxylic acids are
- Benzoic acid
- 2-hydroxy benzoic acid
Uses of ethanoic acid
- It is used in making compounds like cellulose ethanoate, dyes etc.
- It is used as an organic solvent.
- It is used in the food industries for preserving and flavouring food.
- Used for coagulating rubber latex.
Evaluation
- Give three (3) classes of alkanoic acid.
- State four (4) uses of ethanoic acid.
Alkanoates
General molecular formula
The alkanoates are called esters. They have a general molecular formula of RCOOR’.
Nomenclature
The naming of alkanoates is obtained by substituting “e” ending in an alkane with “oates” in alkanoates.
Preparation e.g. ethyl ethanoate.
Ethyl ethanoate is prepared by the esterification between ethanol and glaciusethanoic acid at 1500C in the presence of concentrated tetraoxosulphate (VI) acid
C2H5OH + CH3COOH → CH3COOC2H5 + H2O
Physical properties
- Ethyl ethanoate is a colourless volatile liquid with a pleasant smell.
- It is slightly soluble in water.
- It has a boiling point of 750C.
Chemical properties
Hydrolysis:
Ethyl ethanoate can be hydrolysed by water to produce ethanoic acid and ethanol.
CH3COOC2H5 + H2O → CH3COOH + C2H5OH.
N.B: If an alkali is used instead of water, it will produce the salt of the acid e.g.
CH3COOC2H5 + NaOH → CH3COONa + C2H5OH
Reaction with ammonia:
Ethyl ethanoate reacts with ammonia to produce ethanol and then amide
CH3COOC2H3 + NH3 → C2H5OH + CH3COOH2
Reduction:
Ethyl ethanoate can be reduced by hydrogen from lithium tetrahydridoaluminate (III) as a reducing agent
CH3COOC2H5 + 4[H] → 2C2H5OH
Uses of alkanoates/esters
- They are used as food flavours.
- Used in perfumes and cosmetics
- Used as a solvent for cellulose nitrate.
- Used for quick-drying substances like paints, nail varnishes etc.
General evaluation
- Write the general structure of the ester.
- Write a balanced equation for the reaction between propanol and butanoic acid.
(a) Name the products formed.
(b) What type of reaction is involved.
Reading assignment
New School Chemistry by Osei Yaw Ababio page.504-509
Weekend assignment
- The name of (CH3)2 CHCOOH is A. Propane acid B. 2-methylhutanoic acid C. Dimethyl butanoic acid D. Propanoic acid
- Citric acid appears in unripe orange while ethanoic acid appears in A. Unripe pawpaw B. Carrot C. Vinegar D. Rice
- Esters are employed in the following except. A. Making perfumes B. Making cement C. Nail varnishes D. Making yeast
- An alkanoic acid reacts reversibly with an alkanol to produce. A. a salt B. an ester C. a sugar D. an alkene
- Ethan-1, 2-dioe acid is A. a mineral acid B. dicarboxylic acid C. citric acid D. a soap
Theory
1a. Give the formula of ethanoic acid and indicate its functional group.
b. Ethanoic acid reacts with both sodium hydroxide and ethanol, suing equations to compare the reactions and classify the products.
2a. Ethylethanoate reacts with both water and alkali; using an equation to compare the reaction.
b. What happens when ethanoic acid is heated strongly with soda-line.
In our next class, we will be talking about Fat and Oil as Higher Esters. We hope you enjoyed the class.
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
