Back to: CHEMISTRY SS3
Welcome to class!
In today’s class, we will be talking about saturated hydrocarbon – alkanes. Enjoy the class!
Saturated Hydrocarbon – Alkanes
Content:
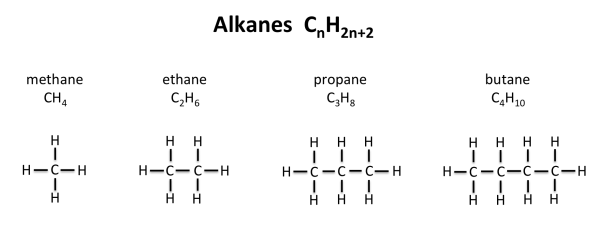
Alkanes e.g. methane (CH4)
- Preparation – properties – Uses
- Isomerism
- IUPAC Nomenclature
Saturated hydrocarbons
Saturated hydrocarbons are hydrocarbons consisting of carbon chains with a single bond between them in which carbon joins with another carbon by single covalent bond e.g. Alkanes (like ethane C2H6, propane C3H8).

Alkanes e.g. Methane (CH4)
The alkanes are aliphatic hydrocarbons. They form a homologous series of saturated hydrocarbons with a general molecular formula of CnH2n+2
Evaluation
- What is saturated hydrocarbon?
- Name one example of alkanes.
Preparation of methane (CH4)
Methane is prepared in the laboratory by heating ethanoate salt with corresponding alkalis e.g. Sodium ethanoate and soda-lime.
Physical properties
- Methane is a colourless and odourless gas
- It is slightly soluble in water.
- It is less dense than air
- It has no action on litmus paper
Chemical properties
- Combustion: Methane burns in air or oxygen to produce steam, carbon(iv) oxide and a lot of heat
CH4 + 2O2 → 2H2O + CO2.
The general equation of alkanes for combustion is represented as
CxHy(g) + ( x + y/4)O2 → y/ 2H2O(g) + xCO2 (g).
- Substitutional reaction: With chlorine gas and bromine gas usually in the presence of ultra’-violet light ( as a catalyst).
Uses
- Methane is used as fuel
- It is used for making hydrogen gas
- It is used in making carbon black
- Used in making carbon (iv) sulphide
- It is used in making hydrocyanic acid
- The derivatives of methane e.g. trichloro-methane can be used as an anaesthetic in a surgical operation. Tetrachloromethane is an organic solvent.
Evaluation
- State four (4) uses of methane
- Identify two (2) chemical properties of methane.
Isomerism
This is the existence of two or more organic compounds, (isomers) with the same molecular formula but different structures.
- Isomers are compounds having the same molecular formula but different structural formula.
- As the number of carbon atoms in a molecule increases, the number of isomers also increases, for example, pentane has the following three isomers.

Types of isomerism
- Structural Isomerism
- Stereoisomerism.
Structural isomerism:
The structural isomerism occurs in organic compound with the same molecular formula but a different structural arrangement of the carbon atom.
Types of structural isomerism
- Chain isomerism: This is the kind of isomerism which occurs due to the differences in the way by which the carbon atoms are arranged in the chain.

- Functional isomerism: This is the kind of isomerism which due to the difference in the functional group.
e.g.

- Positional isomerism: This is the kind of isomerism which occurs as a result of the difference in the way the functional group is positioned.
Example:

Stereoisomerism:
Types of stereoisomerism
- Geometric Isomerism: This is the existence of a compound with the same molecular formula but different spatial structural formula. E.g.

- Optical Isomerism: They have different configuration and they rotate plane-polarized light e.g.

IUPAC Nomenclature
In IUPAC nomenclature, every name of the organic compound consists of a ROOT, SUFFIX and PREFIX names.
-
Root name:
Name of the parent aliphatic hydrocarbon with the longest carbon chain in a molecule.
-
Suffix name:
Name of the principal functional group substituents on the longest carbon chain in a molecule.
-
Prefix name:
Name of the other substituents on the longest carbon chain which are not functional group e.g. 1-chloroethan– 1-ol.
1-chloro = Prefix
ethane = root
-1- ol Suffix
Rules for IUPAC nomenclature
- Select the longest continuous carbon chain as parent hydrocarbon.
- Number the longest carbon chain. Starting from the end that gives the lowest possible number to the suffix and then the prefix.
- Indicate the other substituents by prefixes, preceded by a number to show their position on the carbon chain.
- If the same alky or other substituents group occur more than once as side chain show how many are present by using prefix di, tri, tetra, etc. and indicate by various number the position of each group on the longest carbon chain.
- If there are several different alkyl groups attached to the present chain, name them in order of increasing size or in alphabetical order.

6. If there are halogens together with alkyl groups attached to the parent chain, name the halogen first in alphabetical order and the alkyl group as explained earlier.

General evaluation
- Name the following compound: CH3CHClCH2OH
- List the rules for IUPAC Nomenclature.
Weekend assignment
- The structure of 1,2,3-trichloro-2-methyl butane is
- Which of these compounds exhibits resonance? (a) Ethanol (b) Ethane (c) Benzene (d) Ethyne.
- The solubility of alkane in an organic solvent is due to the presence of its (a) polar molecule (b) non-polar molecule (c) Low melting points (d) low freezing points.
- Which of these is an alkane member? (a) Ethene (b) Pecane (c )Propyne (d) Butanol
Theory
1. Write briefly on the following:
- Cracking
- Isomerism
- Homologous Series
- Stereo Isomerism
- Optical Isomerism.
2. Name the following compound
- CH2(OH)CH(OH)CH2OH
- C2H4CL2
In our next class, we will be talking about Unsaturated Hydrocarbon – Alkenes. We hope you enjoyed the class.
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
School Owner? Automate operations, improve learning outcomes and increase your income with Afrilearn SMSGet more class notes, videos, homework help, exam practice on Android [DOWNLOAD]
Get more class notes, videos, homework help, exam practice on iPhone [DOWNLOAD]
Ok I love this platform it helps to widen our knowledge on things we haven gone through in our studies and it also helps us to learn further viewers come and learn it will help you in life
Yes, this platform is fun for learning.
This platform helps. Me a lot
Well, I love this. Though I still don’t understand the rules for IUPAC nomenclature. I hope you can shed more light on this.
This app is new to me and i think it great.