Back to: BASIC SCIENCE JSS2
Welcome to Class !!
We are eager to have you join us !!
In today’s Basic Science class, We will be Explaining some phenomena using Kinetic Theory. We hope you enjoy the class!

TOPIC: EXPLANATION OF SOME PHENOMENA USING KINETIC THEORY
The present form of the kinetic theory has existed for more than 100 years. Some of the things it helps to explain will be described in this lesson. The kinetic theory of matter explains the following scientific phenomena:
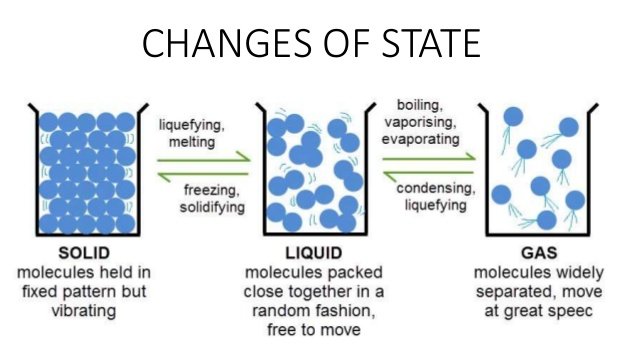
CHANGES OF STATES OF MATTER
An important part of the kinetic theory is that the hotter particles get, the faster they move. Some properties of solids, liquids and gases are:
- A solid has a fixed volume and a fixed shape. This is because the particles in a solid cannot change their position.
- A liquid has a fixed volume, but it takes the shape of any container. The volume is fixed because the particles remain together in a crowd or group, but the shape of the crowd can change because the particles can move.
- A gas has no fixed volume or shape. It expands to fill any container. This is because the particles in a gas do not remain together and are completely free to move anywhere.
When a solid (ice) is heated, the particles vibrate more strongly as they gain kinetic energy they start pushing past one another. The forces of attraction between them weaken and when this happens, the solid melts into water. On heating, the molecules will have enough kinetic energy to escape from the attractive forces holding the molecules together and thereby turn to steam. The molecules of ice, liquid water, steam or water vapour are the same. The differences are how they are arranged, their average kinetic energy and how strongly they are held together by intermolecular forces.
RELATIONSHIP BETWEEN PRESSURE, VOLUME AND TEMPERATURE
If gas is enclosed in a container, it exerts pressure on the walls of the container. The kinetic theory of matter explains gas pressure as the total force exerted by gas molecules colliding against the walls of a container. If a container can expand, like with a balloon or cylinder and piston, increasing the pressure can increase the volume. Like, the balloon will get bigger. Also, if you increase the temperature of the gas- and thus the kinetic energy of its molecules, you increase the pressure or the volume of the container.
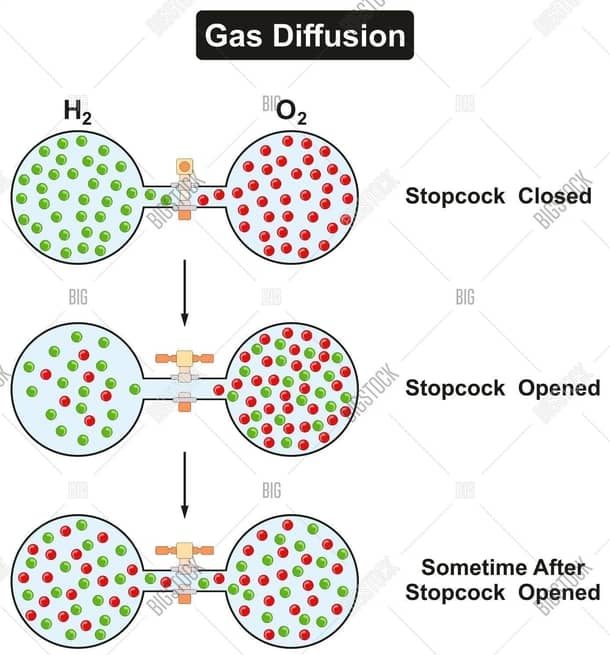
DIFFUSION IN GASES
Sometimes your nose tells you what is for dinner before you even see it! The smell diffuses (spreads out) through the air, from your dinner to your nose. Diffusion is responsible for the spread of odours even without any air disturbance. The kinetic model makes diffusion easy to explain. Molecules evaporate from anything that smells and fly about between the particles of air. Some of the molecules from the food reach your nose. An example of diffusion that you can see is smoke diffusing through the air. Diffusion is faster in gases than liquids where there is more space for them to move while diffusion is negligible in solids due to the close packing of the particles.
DISSOLVING AND DIFFUSION IN LIQUIDS
The kinetic model helps to explain what happens when a solid dissolves in a liquid. When a little sugar or salt is added to water, the molecules of water bump against the sugar or salt crystals. Some of the sugar or salt molecules are knocked off the crystals (they dissolve) and are carried away in the jostling crowd of water molecules (they diffuse). After some time, all the sugar and salt molecules dissolve and diffuse throughout the water. Heat speeds up dissolving and diffusion because heat makes the water molecules move faster.
We have seen now how the kinetic theory is applicable to different phenomena.
We have come to the end of this class. We do hope you enjoyed the class?
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
In our next class, we will be talking about Boiling and Evaporation. We are very much eager to meet you there.

Where is the explanation of some phenomena using kinetic theory
Please show it🙏