Back to: BASIC SCIENCE JSS2
Welcome to the JSS2 Third Term!
We have covered a lot during the course of this class and now in this final term, we will build more on our work from previous terms.
In today’s class, We will be discussing Thermal energy flow. Please Join Us and Enjoy the class!

TOPIC: THERMAL ENERGY 1 – HEAT FLOW
What exactly is heat? Heat is a form of energy called thermal energy. Thermal energy is the energy resulting from the motion of particles. It is a form of kinetic energy and is transferred as heat. It is used for a lot of things like heating in industries and cooking our food. We need heat to keep warm, to cook our food and for other human activities. Where does the heat come from? Our most important source of heat is the sun. The Sun is the primary source of all energy available in the world and this energy from the Sun reaches us in the form of light and heat. Electric cookers, electric heaters and electric light bulbs all get hot – so do many machines such as the engine of a truck. Even our bodies make heat. You can feel the warmth of your own breath when you breathe out on a cold day. Fire is a good source of heat too. Specifically, we will be considering the transfer of heat energy in this lesson.
All forms of matter, whether a solid, liquid or gas, are composed of atoms or molecules in constant motion. Due to this constant motion, all atoms have thermal (heat) energy. Whenever a substance is heated, the atoms move faster and faster. When a substance is cooled, the atoms move slower and slower. An object has more thermal energy when it is warm than when it is cool. In the simplest of terms, the discipline of heat transfer is concerned with only two things: temperature and the flow of heat.
Any source of heat warms up nearby objects and these become secondary sources of heat. If we get close to a hot object, we can feel the heat coming from it. In many traditional cultures, stones are heated in a fire, and then heat from the stones is used to cook food. Heat flows from a hot body to a cold body by itself. If we place a body at a higher temperature in contact with a body at a lower temperature, we notice after a while that the body at a higher temperature loses some heat to the body at a lower temperature. This process of heat flow will continue until the two bodies attain a final steady temperature (equilibrium temperature).
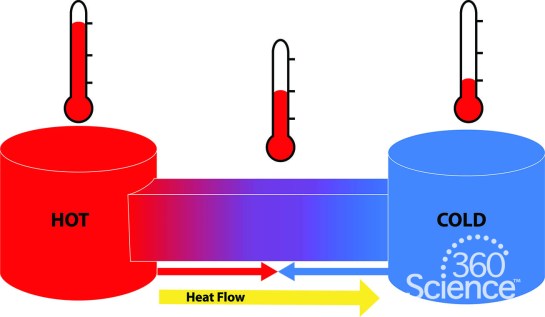
When a difference in temperature exists between materials, the heat flow can only be slowed, not stopped. Also, if there is a temperature difference in a system, heat will always move from higher to lower temperatures. This transfer of heat energy can happen by three main methods, which are conduction, convection and radiation. These methods of heat transfer will be considered in the next lesson.
That brings us to the end of this class. We do hope you enjoyed this class. Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
Our next class we will be discussing Thermal Energy – Heat Transfer. See you there!!
Get more class notes, videos, homework help, exam practice on Android [DOWNLOAD]Get more class notes, videos, homework help, exam practice on iPhone [DOWNLOAD]