Back to: BASIC SCIENCE JSS2
Welcome to Class !!
We are eager to have you join us !!
In today’s Basic Science class, We will be discussing the Kinetic Theory. We hope you enjoy the class!

TOPIC: KINETIC THEORY
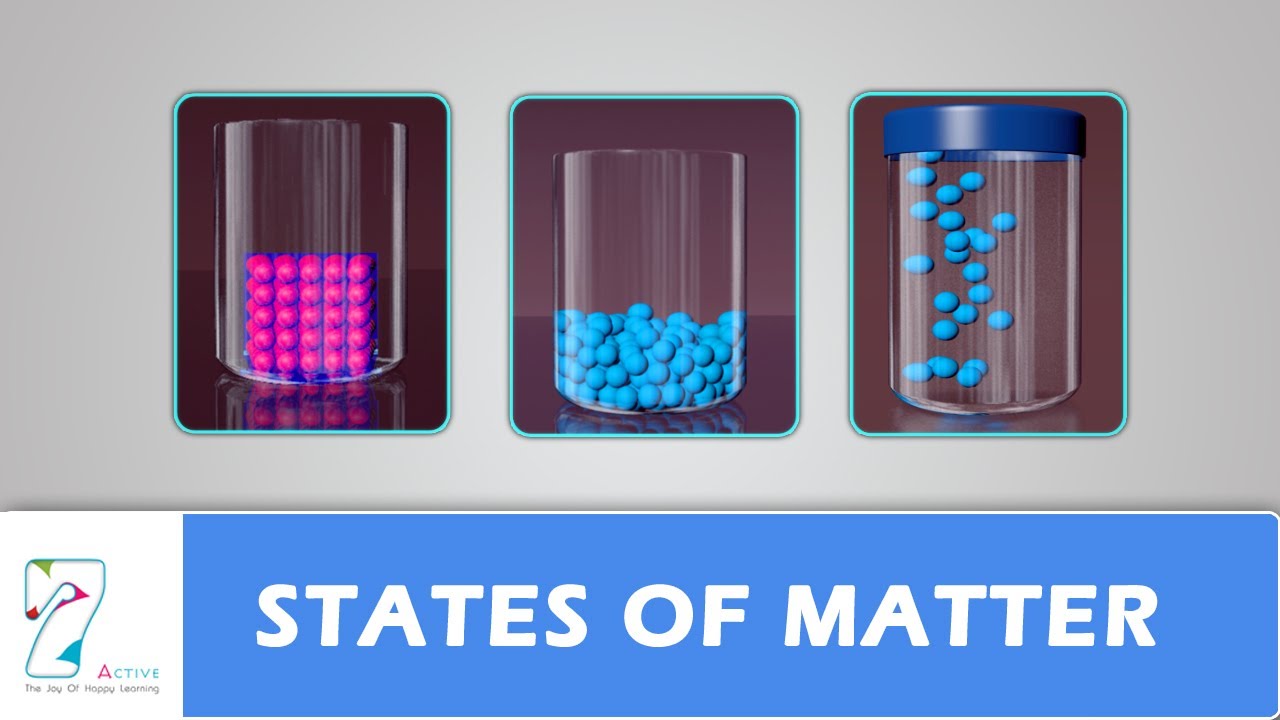
Matter is anything that has mass and takes up space. Anything around us and in the entire universe can be classified as either matter or energy. Matter is composed of tiny particles called atoms. In some substances, the atoms are joined together in small groups called molecules. Atoms are very, very tiny. They are much too small to be seen, even with the best microscopes. The kinetic theory of matter is a prediction of how matter behaves, based on certain assumptions and approximations. The kinetic theory is a model that shows how particles are arranged, and how they behave, in solids, liquids and gases. The particles in the kinetic model may be atoms or they may be molecules (groups of atoms joined together). The particles have energy, so they can move.
In solids, the particles are arranged in regular patterns. They have enough energy to vibrate, but they do not change position. This type of vibrational movement is why a solid won’t change shape no matter what kind of container you put it in. In liquids, the particles are crowded close together but they are always on the move, like people jostling in a crowd. In gases, the particles are far apart. The particles of a gas are so far apart that the attractive forces between them are assumed to be negligible. They have lots of energy and fly around at high speed.
According to the kinetic theory of matter,
- Matter is made up of tiny particles (atoms and molecules)
- Particles of matter are in constant random motion irrespective of the phase they are. This motion is different for each of the three states of matter. They are colliding with each other and the walls of their container. When the molecules collide with each other, or with the walls of a container, there is no significant loss of energy.
- Particles of matter are held together by very strong electric forces.
- The molecules of matter always attract each other due to the intermolecular force of attraction.
- There are empty spaces between the particles of matter that are very large compared to the particles themselves.
- Each substance has unique particles that are different from the particles of other substances.
- Temperature affects the speed of the particles. The higher the temperature, the faster the speed of the particles. Absolute zero is the temperature used to describe when all movement is as slow as it can possibly.
As well as being in continuous motion, molecules also exert strong electric forces on one another when they are close together. The force of attraction holds molecules together while the force of repulsion causes matter to resist compression between molecules. The kinetic theory can explain the existence of the solid, liquid and gaseous states.
We have come to the end of this class. We do hope you enjoyed the class?
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
In our next class, we will be Explaining some phenomena using Kinetic Theory. We are very much eager to meet you there.
