Back to: PHYSICS SS2
Welcome to class!
In today’s class, we will be talking about the measurement of heat energy. Enjoy the class!
Measurement of Heat Energy

Heat energy is the energy that is transferred from one place another as a result of temperature difference. Heat energy always flows from a region of high temperature to the region of lower temperature. Heat energy is called thermal energy. It is measured in joule.
Heat Capacity:
Heat capacity is the quantity of heat energy that is required to raise the temperature of a given mass of an object by one Kelvin. The unit of heat /thermal capacity is joule per Kelvin (J/K or JK-1)
The formula of Heat Capacity, H:
The formula for calculating heat capacity is as stated:
Heat Capacity Cp = mass (m) x Specific heat capacity (c)
Cp = m x c. (Cp is also called thermal capacity)
Calorimeter:
A calorimeter is an instrument used in measuring the quantity of heat. It is of four types namely
- Bomb calorimeter
- Glass calorimeter
- Ice calorimeter
- Regnant calorimeter
The law of Calorimeter states that heat lost by hot substance = heat gained by the cold substance. Lagging is defined as the process of reducing heat hot to the surrounding.
WORKED EXAMPLES
(1) Calculate the heat capacity of a mass 258g if its specific heat capacity is 900JKg-1K-1.
SOLUTION
Data given in the question:
Mass = 258kg, specific heat capacity, c = 900Jkg-1K-1
Formula: heat capacity = mass x specific heat capacity
Substitution: Cp = 258 x 900
Cp = 232200JK-1
(2) The thermal capacity of an object is 585 JK-1. Calculate the mass of the object if its specific heat capacity is 390 kg-1K-1.
SOLUTION
Data given in the question:
Heat capacity =585 JK-1,
Specific heat capacity is 390 kg-1K-1
Formula: thermal capacity Cp = mass * specific heat capacity
Substitution: 585 = mass x 390
Make mass the subject: Mass = 585 ÷ 390.
Mass = 1.5 Kg
Specific Heat Capacity
Specific heat capacity of a substance is the quantity of heat that is required to raise the temperature of 1 kg mass by 1°C or 1Kelvin.
The formula of Specific Heat Capacity:
The quantity of heat that is required to raise the temperature of a substance by 1 K is directly proportional to the mass of the substance, the temperature changes the substance.
Mathematically,
Quantity of heat H Mass of substance x temperature change
H = c x m x ∆
If we make c the subject t,
H is the quantity of heat measured in Joule, m is mass of substance measured in kg,
is temperature measured in °C or K.
The unit of specific heat capacity is Joule per Kilogram per Kelvin (JK-1K-1)
WORKED EXAMPLES
(1) What is the amount of heat that is required to raise the temperature of 350 g of the aluminium cone from 30°C to 68°C if its specific heat capacity is 900 JK-1 K-1.
SOLUTION
Data given in the question:
Mass = 350 g = 350 ÷ 1000 = 0.35 kg,
s.h.c = 900 JK-1 K-1,
1 = 30°C, 2 = 68°C
∆ = 2 – 1 = 68 – 30 = 38°C
Formula: Q = m x c x ∆
Substitution: Q = 0.35 x 900 x 38
Q = 11970 Joules
(2) What is meant by the statement that the specific heat capacity of water is
4200JK-1K-1? Calculate the temperature change when 1000 J of heat is supplied to 100g of water.
SOLUTION
Data given in the question:
Mass = 100g = 100 ÷ 1000 = 0.1 kg,
c = 4200JK-1K-1,
Q = 1000 J
Formula: Q = m x c x ∆
Substitution: 1000 = 0.1 x 4200 x ∆
Make ∆ the subject:
∆ = 2.38°C
METHODS OF DETERMINING THE SPECIFIC HEAT CAPACITY OF A SUBSTANCE:
Different methods can be used to determine the specific heat capacity of a
Substance. The specific heat capacity of a substance can be determined by the following methods:
- Electrical method.
- Method of mixtures
Determination of Specific Heat Capacity of a Solid by Electrical Method
In the electrical method of determining the specific heat capacity of a solid substance, the electric heater is used to provide the heat required for the experiment.
Aim: To determine the specific heat capacity of a solid.
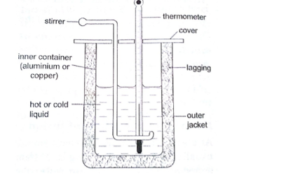
Apparatus
Solid whose specific heat capacity is o be determined, heater, voltage source,
Thermometer and Calorimeter.
Setup Diagram:
Procedures
Get a solid whose specific heat capacity you want to determine and that fits into the calorimeter. Bore two holes in the solid. Weigh and record the mass of the solid. Insert the thermometer and the heater into the holes and add little oil to help establish good thermal contact between the block and the thermometer and heater. Read and record the initial temperature of the solid and calorimeter. Switch on the electrical heater so that current flow for some times until the temperature rise is about 15°C. Use a stopwatch to measure the time for which current flows. Read and record the final temperature of the solid and thermometer.
Data from the Procedures:
Mass of solid = Ms
Mass of calorimeter = Mc
The initial temperature of solid and calorimeter = 1
The final temperature of solid and calorimeter = 2
The voltage applied across heater = V
Current that flow = I
Time for which current flow = t
Theory of calculation:
Heat supplied by heater = heat gained by solid + heat gained by the calorimeter.
Formula:
Introducing the formula of the quantity of heat for each of them as stated above, we have:
IVt = Ms x Cs x ∆ + Mc x Cc x ∆
Ms = mass of solid.
Cs = specific heat capacity.
Mc = mass of calorimeter.
Cc = specific heat capacity of the calorimeter.
∆ = temperature change.
I = current.
V = voltage. t = time.
At this point, you make the variable that you want to calculate the subject of the formula.
Precautions
- Make sure that the calorimeter is lagged to prevent heat loss.
- Take the reading when the mercury thread is steady.
WORKED EXAMPLES
(1) An electric heater, rated 20V, 40 W, fitted into a metal block supplied heat to the block of mass 2.25 kg and specific heat capacity of 460JK-1K-1. Calculate the temperature rise in the block if the current flow for 10 minutes.
SOLUTION
Data given in the question:
Voltage = 20 V, Power = IV = 40 W, mass =2.25kg, C = 460 JK-1K-1
Time = 10 minutes = 10 x 60 = 600 seconds.
Note that the mass, specific heat capacity of the calorimeter is not mentioned in the question. Therefore, you have to ignore the quantity of heat aspect of the calorimeter.
Formula: IVt = Ms x Cs x ∆ + Mc x Cc x ∆
IVt = Ms x Cs x ∆
P x t = Ms x Cs x ∆
Substitution: 40 x 600 = 2.25 x 640 x ∆
∆ = 16.6°C
Determination of Specific Heat Capacity of a Liquid by Electrical Method
Heat supplied by heater = heat gained by liquid + heat gained by the calorimeter.
Formula:
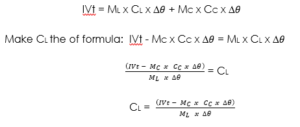
ML = Mass of liquid.
CS = Specific heat capacity.
MC = Mass of calorimeter.
CC = Specific heat capacity of the calorimeter.
∆ = temperature change.
I = current.
V = voltage. t = time.
In our next class, we will be talking about Latent Heat. We hope you enjoyed the class.
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.

It is a nice place to browse any difficult topic
in the first example of heat example, I saw 258g why wasn’t it converted to kilogram first beforeproceeding to solving?