Back to: PHYSICS SS2
Welcome to class!
In today’s class, we will be talking about the measurement of temperature. Enjoy the class!
Measurement of Temperature

Measurement of Temperature has been discussed intensively in the previous lesson. However, a recap and calculation on the topic will be review in this lesson. The instrument that is used to measure temperature is called thermometer. The thermometers used any physical property of a substance which varies with temperature and is easily measurable as means measuring temperature.
Thermometric Substances:
Thermometric substances are substances whose physical properties are used in the construction of thermometers.
Types of Thermometers, Thermometric Substances and physical properties
Type of thermometer:
- Liquid in glass:
Thermometric substance: Mercury or Alcohol.
Physical property: Change in volume of liquid with temperature.
- Gas thermometer:
Thermometric substance: Gas.
Physical property: Gas pressure changes with temperature.
- Resistance thermometer:
Thermometric Substance: Resistance wire
Physical Property: Electrical resistance change with temperature.
- Thermocouple:
Thermometric Substance: Two dissimilar wires
Physical Property: Change in electric potential difference between two metal junctions at different temperatures.
- Bimetallic thermometer:
Thermometric Substance: Two dissimilar metals
Physical Property: Different expansion of the bimetallic strip.
Temperature Scale of Thermometer:
A thermometer has two reference temperatures or fixed points. They are the Upper Fixed Point and the Lower Fixed Point.
The Upper Fixed Point:
The upper fixed point is the temperature of steam from pure water boiling at standard atmospheric pressure of 760 mm of mercury (760mmHg).
The Lower Fixed Point:
The lower fixed point is the temperature of pure melting ice block at standard atmospheric pressure of 760mm of mercury, (760mmHg).
Fundamental Interval
The fundamental interval is the difference between the upper fixed point and the lower fixed point of a thermometer. The calibration of the fundamental interval of a thermometer depends on the temperature scale chosen.
The formula for Calculating Fundamental Interval:
Fundamental Interval = upper fixed point – lower fixed point.
Therefore,
Fundamental interval of degree Celsius = 100° – 0° = 100°C
Fundamental interval of Kelvin scale = 212 – 32 = 180K
Fundamental interval of Fahrenheit scale = 373 – 273 = 100°F
Types of Temperature Scales
In temperature measurement, three types of scales are currently used in measuring temperature. They are:
- The Celsius scale
- The Fahrenheit scale
- The Absolute or thermodynamic or kelvin scale
Diagram Of Temperature Scale
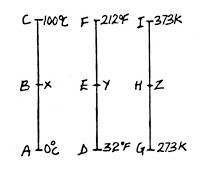
The figure shows the three different temperature scales, in °C, °F and K, that are used in measuring temperature.
Conversion Of Temperature From One Scale To Another
- Conversion of temperature from degree Celsius (°C) to degree Fahrenheit ( °F ):
From the figure above, I will show and explain how to convert temperature from degree Celsius to degree Fahrenheit as follow:
AB/AC = DE/DF
(X – 0)/(100 – 0) = (Y – 32)/(212 – 32)
X/100 = (Y – 32)/180
(X/100)*180 = Y – 32
1.8*X = Y – 32
Note:
Where X is Temperature in degree Celsius while Y is temperature in degree Fahrenheit.
AB mean distance from A to B. AC means distance from A to C. DE means distance from D to E. DF means distance from D to F, and so on.
Worked Examples:
1.Change 25°C to temperature in Fahrenheit.
Solution
Data given in the question:
Temperature in °C (X) = 25°C. Y = ?
Formula: 1.8 x X = Y – 32
Substitution: 1.8 x 25 = Y – 32
Make Y the subject: 1.8 x 25 + 32 = Y.
Y = 45 + 32.
Y = 77°F
2.Convert 120°F to degree Celsius.
Solution
Data given in the question:
Temperature in °F, Y = 120°F
Formula: 1.8 x X = Y – 32
Substitution: 1.8 x X = 120 – 32.
1.8 x X = 88
Make X the subject: X = 88 ÷ 1.8.
X = 48.89°C
Note: you can equally solve the problems using the concept I used to obtain the formula. This can be done by simply substituting for X and Y respectively in the process and then follow the procedures to derive at the answers.
Conversion of temperature from degree Celsius (°C) to Kelvin (K):
Also, from the figure above, I will explain how to convert temperature from degree Celsius to temperature in Kelvin. This is illustrated in formula 2.
Note: X is temperature in degree Celsius while Z is temperature in Kelvin.
AB/AC = GH/GI
(X – 0)/(100 – 0) =
(Z – 273)/(373 – 273)
X/100 = (Z – 273) / 100
100 cancels 100: X = Z – 273 or Z = X + 273
Where X is temperature in degree Celsius (°C) while Z is temperature in Kelvin (K)
WORKED EXAMPLES
(1) Calculate the value of 200°C in Kelvin.
SOLUTION
Data given in the question:
Temperature in °C, X = 200°C.
Formula: X = Z – 273
Substitution: 200 = Z – 273
Make Z the subject: 200 + 273 = Z
Z = 473 Kelvin
(2) Given that the temperature of a body is 527kelvin, determine this value in degree Celsius.
SOLUTION
Data given in the quest:
Temperature in Kelvin, Z = 527K.
Formula: X = Z – 273
Substitution: X = 527 – 273.
X = 254°C
Or
You can equally solve the problems using the concept I used to obtained the formula. You do this by substituting for X and Z respectively in the procedures and the follow the procedures to derive at he answers.
Thus:
From the question, Z = 527K.
Substitute for Z in the procedures
B/AC = GH/GI
(X – 0)/(100 – 0) = (Z – 273)/(373 – 273)
. X/100 = (Z – 273) / 100
(X – 0)/(100 – 0) = (527 -273)/(373 – 273)
X/100 = ( 527 – 273 ) / 100
X = 527 – 273 = 254°C
Conversion of temperature from degree Fahrenheit (°F) to Kelvin (K):
Also, from the figure far above, I will explain how to convert temperature from degree Fahrenheit to temperature in Kelvin. This is illustrated in formula 3.
From the figure,
DE/DF = GH/HI
(Y – 32)/(212 – 32) = (Z – 273)/(373 – 273)
. (Y – 32)/180 = (Z – 273)/100
. Y – 32 = 180 x (Z – 273)/100
. Y – 32 = 1.8 x (Z – 273)
WORKED EXAMPLES
(1) It was recorded that the temperature of a body was 320°F. Determine the value of this temperature in Kelvin.
SOLUTION
Data given in question:
Temperature in Fahrenheit, Y = 320°F
Formula: DE/DF = GH/GI
(Y – 32)/(212 – 32) = (Z – 273)/(373 – 273)
Substitution:
(320 – 32)/180 = (Z – 273)/100
. 288/180 = (Z – 273)/100
Make Z the subject: 288÷180 x 100 = Z – 273
28800 ÷ 180 = Z – 273
. 160 = Z – 273
160 + 273 = Z.
Z = 433K
(2) Convert 385K to temperature in degree Fahrenheit.
SOLUTION
Data given in the question:
Temperature in kelvin, Z = 385K
Formula: DE/DF = GH/GI
(Y – 32)/(212 – 32) = (385 – 273)/(373 – 273)
Substitution:
(Y – 32)/180 = 112/100
. (Y – 32)/180 = 1.12
Y – 32 = 1.12 x 180
. Y – 32 = 201.6
Y = 201.6 + 32.
Y = 233.6°F
Relationship Between Temperature in Degree Celsius and Kelvin
The difference between temperature in degree Celsius and Kelvin is the formula that connect the two scales. The formula is as stated below.
Temperature in Kelvin T = temperature in degree Celsius + 273
That is,
T = ( + 273)K
WORKED EXAMPLES
(1) What is the value of 35°C in Kelvin?
SOLUTION
Data given in the question:
Temperature in degree Celsius = 35°C
Formula: temperature in Kelvin = + 273
Substitution: T in Kelvin = 35 + 273.
T in kelvin = 308 Kelvin
2.Change 356K to temperature in degree Celsius
SOLUTION
Data given in the question:
Temperature in Kelvin = 356K
Formula: Temperature in Kelvin = + 273
Substitution: 356 = + 273
Make the subject: = 356 – 273 ➡ = 83°C
3.Convert – 120°C to temperature in Fahrenheit.
SOLUTION
Data given in the question:
Temperature in degree Celsius = -120°C
Formula: Temperature in Kelvin = + 273
Substitution: T in Kelvin = – 120 + 273. ➡ T = 153 Kelvin
In our next class, we will be talking about Measurement of Heat Energy. We hope you enjoyed the class.
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.

I need classes of thermometer
Glad you found it helpful😊 For even more class notes, engaging videos, and homework assistance, just download our Mobile App at https://play.google.com/store/apps/details?id=com.afrilearn. It’s packed with resources to help you succeed🌟
i love this class
I love this class 🥰🥰