Back to: PHYSICS SS3
Welcome to class!
In today’s class, we will be talking about quantum. Enjoy the class!
Quantum

Concept of energy quantization
The energy of electrons consists of small units or discrete packets called quanta of amount, hv, where v is the frequency of radiation and h is a constant known as the Planck constant. Thus, the kinetic energy of electron = hv
The energy level in an atom
The energy level of different atoms is different because of the numbers of an electron in their outermost shell. The energy of the electron can be changed from one energy level to another by disturbing the atom.
The energy level in hydrogen
Ground state energy level Eo:
This is the lowest energy level of an electron in an atom.
Excited-state energy level En:
This is the energy level attain by an electron in an atom when the electron is disturbed.
Excited energy or excitation energy:
This is the minimum amount of energy that is required to raise the energy level of an atom from the ground state to an excited state.
The formula for calculating energy level in an atom
E = En – Eo. Where Eo is the energy level of the electron in the ground state. En is the energy level of the electron in the excited state and E is the energy difference between the energy level of the ground state and the excited state.
Energy level chart:
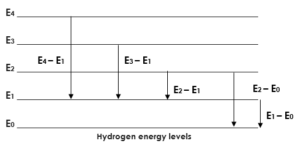
N = 1, 2, 3, 4, 5 ,…….
N represents the energy level. Each number represents a particular energy level.
The value of the energy level of an atom
The ground state energy level for hydrogen = – 13.6 Ev.
Recall that 1 eV (electron volt) = 1.06 x 10⁻¹⁹ Joule.
Therefore, -13.6 eV = -13.6 x 1.06 x 10⁻¹⁹ Joule.
= -21.76 x 10⁻¹⁹ Joule.
The formula for calculating other energy levels of an electron in an atom
En = where n represents the electron quantum number
Worked examples
(1) Calculate the energy level of an electron when n = 2
Solution

(2) Find the energy level between En = 3 and 4
Solution
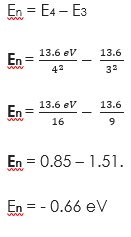
Emission of radiation or spectra
When an atom is heated or excited the electron in the atom gain energy and move from its ground state (lower energy level) to a higher state (excited energy level). When the disturbance or heat is removed, the electron returns back to its ground state and the electron releases energy in the form of radiation or light
Photon or quanta
It is a packet of radiation energy that is emitted by a body when its temperature is increased or lowered.
The formula for calculating the energy of quanta or photon
E = hf.
F is the frequency of radiation of photon. Then h is called Planck constant.
Spectra is the excess energy in the form of radiation emitted by a particle when the electron is returning from excited to a ground state
Types of Spectra
(1) Emission Spectra
These are spectra observed due to t the light emitted when the temperature of an atom, gas or metal is raised.
(ii) Line Spectra
Line spectrum is obtained from atoms in gases such as hydrogen or neon at low pressure in a discharge tube. This spectrum mainly occurs in sodium, mercury vapours and hydrogen gas.
(iii) Continuous spectrum
This spectrum occurs mostly in the sun. it contains all the wavelength from one end to the other. The spectra are formed from the light-emitting from incandescent solid or gas.
(iv) Band spectrum
This spectrum occurs in chemical compound, e.g. hydrogen, oxygen, carbon (IV) oxide. When they are heated, they produce an emission of a discontinuous spectrum of a large number of luminous band. The band hand becomes sharp at one edge but fades off gradually at the other.
(v) Absorption spectrum
These are spectra observed when a beam or rays of light are incidental on a transparent medium.
Equation of radiation energy
Recall that, E = hf and E = En – Eo.
Now let us substitute these formulae into one another, then we will get,
hf = En – Eo. Also, recall that, v = hf (from wave equation)
Let us make f the subject and substitute in the formula above. Therefore,
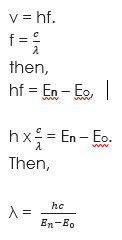
Where h is Planck constant, v is velocity. Eo is ground state energy level and En
is excited state energy level.
Worked examples
(1) An electron jumps from one energy level to another in an atom radiating 4.5 x 10-19 Joules. If Planck’s constant is 6.6 x 10-34Js, what is the wavelength of the radiation? Take velocity of light = (3 x 108m/s).
Solution
Given parameters
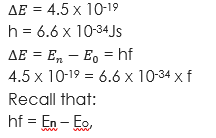
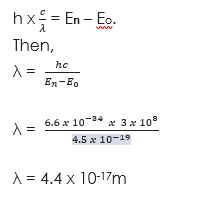
(2) An atom excited to an energy level E2 (- 2.42 x 10-19J) falls to the group state E0 (-21.8 x 10-19J). Calculate the frequency and the wavelength of the emitted photon. (h = 6.6 x 10-34Js)
Solution
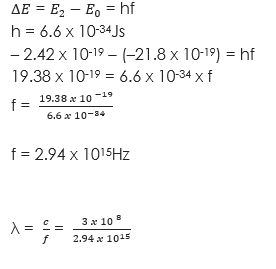
λ = x 10-7m
General evaluation
- Define Excitation Energy
Assignment
(1) (a) A light wavelength 5.0 x 10-7m is incident on metal resulting in photoemission of electrons. If the work function of the metal is 3.04 x 10-19Js calculate the
(i) frequency of the light
(ii) Energy of the incident photo
(iii) Maximum kinetic energy of the photoelectrons (speed of the light = 3.00 x 108m/s; Planck’s constant = 6.6 x 10-34Js)
In our next class, we will be talking about the Photoelectric Effect. We hope you enjoyed the class.
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
