Back to: PHYSICS SS3
Welcome to class!
In today’s class, we will be talking more about the electric field. Enjoy the class!
Electric Field II
Production of continuous charges
A continuous flow of charges is produced from primary and secondary cells.
Electric cell
An electric cell is a device used to generate the force required to move the electrons through the internal and external circuit. There are two types of electric cell. The Primary and Secondary Cells.
Primary cells
The primary cells are cells that cannot be recharged. They give out electrical energy directly from its stored energy. Examples include:
- Simple cell
- Daniel cell
- Leclanche cell
Simple cell (voltaic cell)
A simple cell consists of a glass vessel containing dilute tetraoxosulphate (VI) acid. Two electrodes of copper and zinc are immersed in the electrolyte; the copper rod is a positive electrode, while the zinc rod is a negative electrode. When zinc rod is dipped into dilute tetraoxosulphate (VI) acid, the zinc atoms are absorbed into the solution, forming zinc sulphate and the two atoms of hydrogen carrying two units of positive charges. Then more mobile electrons cling to the zinc rod and this makes it negatively charged. After connection, the hydrogen atoms which are positively charged travel towards the copper rod. The movement is from zinc to copper in an external circuit.
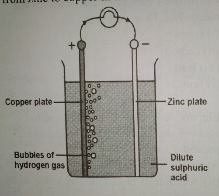
The commercial direction of the current is taken as the flow of positive charge from copper to zinc. The e.m.f. of the cell is maintained by fresh zinc ions going into solution.
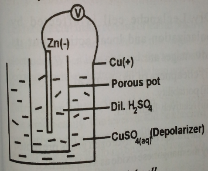
The Daniel cell
It consists of
- a Copper vessel filled with a saturated copper sulphate solution.
- a porous pot containing dilute sulphuric acid (H2SO4) which the electrolyte for this cell.
- an amalgamated zinc rod immersed in the acid. The zinc rod acts as the negative terminal while the copper vessel acts as the positive terminal. The copper sulphate solution acts as depolarizer for the Daniel cell. The initial e.m.f. produced by the Daniel cell is about 1.1 volts and the cell provides only a small current for some time.
The Daniel cell is no longer in use.
The Leclanche cell
It has an e.m.f of about 1.5V. it is made up of:
(a) The wet type
(b) The Dry Leclanche cell.
(a) The wet type
This consists of a zinc rod and the cathode in the solution of ammonium chloride enclosed in a vessel. Carbon is used as the anode. The porous pot is surrounded by manganese dioxide as the depolarizer. The zinc, the carbon and the electrolyte set up an electromotive force which derives a current from zinc to carbon through the cell. The carbon has a higher potential than zinc. the current flows through the outside circuit, from carbon to zinc, but it flows through the inside circuit from zinc to carbon. The wet cell experiences two defects:
(i) Polarization
(ii) It is difficult to carry about without spilling the liquid content.
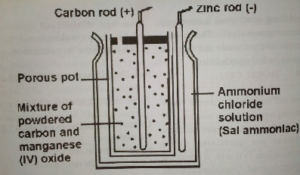
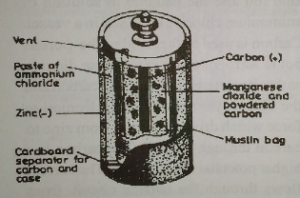
(b) The Dry type
Dry Leclanche cell consists of zinc vessel or can (-ve pole) containing jelly or paste ammonium chloride (NH4CL). Inside the vessel, there is a big round the carbon pole (+ve pole) containing a mixture of manganese (IV) oxide with powdered carbon. Dry Leclanche cell is affected by polarization and local action, but its advantages are:
(i) Cheapness of its chemical
(Ii) Portability
(iii) Relatively high e.m.f.
Secondary cells
There are two types namely:
- The lead-acid accumulator and
- The alkaline or Nickel – Iron (NIFE) accumulator
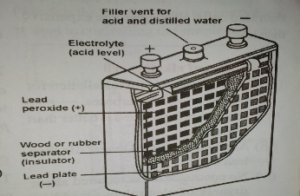
The lead-acid accumulator
The simple form of the cell consists of:
- A positive pole of lead peroxide (chocolate or dark brown in colour)
- A negative pole of the lead plate, (grey in colour) and
- An electrolyte of dilute sulphuric acid
In modern commercial accumulators, both the positive and negative plates are made of grids of lead-antimony alloy, the lead or the lead peroxide. The positive and negative plates containing lead are assembled alternatively in groups and are separated by insulators. All the positive terminal of the accumulator and the negative plates are also connected to form the negative terminal. The whole framework is encased in a plastic container filled with sulphuric acid.
When fully charged, e.m.f. of the cell is about 2.2v and the relative density of the dilute sulphuric acid is about 1.25. the cell is connected completely discharged when the relative density of the acid falls to 1.15.
Lead-acid accumulator
Using or discharging the accumulator
The accumulator is used to supply current to an external resistance e.g. an elastic bulb. After some time of such use, the current will cease to flow and will be indicated by both the ammeter and the bulb. The accumulator is said to be discharged. A little red sulphate is formed at both of the plates and the relative density of the acid and the e.m.f. of the cell decrease below the original levels.
Nickel – Iron (NIFE) Accumulator
The poles of this accumulator are positive nickel plates and negative iron plate. The electrolyte is a solution of potassium hydroxide. The e.m.f. when fully charged is about 1.5 volts and falls to 1.3volts after some time. The NIFE accumulator has a longer life span than the lead-acid accumulator.
Discharging and recharging of an accumulator
To charge an accumulator, we pass direct current to that which is supplies. The positive terminal of the d.c. is connected to the positive terminal of the accumulator and the negative terminal of the d.c. is also connected to the negative terminal of the accumulator. The Rheostat R in the charging circuit is essential as the sulphuric acid has a very low resistance such as 0.02ohm. The ammeter A also included in the circuit to ensure that only the charging current recommended by the manufacturer is used.
Charging the accumulator is considered complete when the relative density of the acid attains the recommended value of 1.25 and the e.m.f. of the accumulator is about 2.2 volts.
Properties of primary cells
- They are not rechargeable
- They are affected by defects such as localization and polarization
- They have a very high internal resistance and hence can give a small current with a very high drop of terminal
Properties of secondary cells
- They are rechargeable
- They are not affected by defects such as localization and polarization
- They have a very low internal resistance and hence can give a small current with a very high drop of terminal
Presentation
- Introduce the lesson by giving them definitions of Electric cell.
- Explain all the definitions by giving examples on each of them.
- Pick out the keywords in the definitions and explain to them.
- Ask them to defined Primary cell by using their own words and correct them if necessary.
- States the properties of Primary and Secondary Cells.
General evaluation
- Name the two advantages which a lead-acid accumulator has over a primary cell.
Assignment
- Draw a circuit diagram for recharging of an accumulator.
- Name the poles and electrolytes for the following:
- Dry Leclanche cell
- Lead-acid accumulator
- NIFE accumulator
In our next class, we will be talking about Electrolysis. We hope you enjoyed the class.
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
